Abstract
Mohs micrographic surgery (MMS) is a specialized surgical and histologic processing technique for cutaneous neoplasms. It combines tumor extirpation and complete surgical margin evaluation. The conceptual basis for this technique is that tumors arising from a single focus have contiguous spread. The chapter reviews the indications, preoperative and postoperative management, commonly treated tumors, the technique itself, and the challenges encountered. The advantages of MMS include observed histological margin control, which leads to superior cure rates, maximum tissue sparing potential, convenience of an outpatient procedure, and cost effectiveness. Preservation of tissue permits more reconstructive options for preserving function and maintaining cosmesis. MMS is a very safe procedure and the complication rates are exceedingly low. However, MMS is one of several modalities for treating cutaneous neoplasms and it is important to use MMS appropriately.
Keywords
Mohs, Mohs micrographic surgery, Mohs surgery, chemosurgery, tissue sparing, margin control, appropriate use criteria for Mohs micrographic surgery, basal cell carcinoma treatment, squamous cell carcinoma treatment
- ▪
Based upon the concept of contiguous spread of a tumor from a single focus
- ▪
100% microscopic tissue margin examination
- ▪
Highest evidence-based cure rate for cutaneous malignancies, but success is dependent on resources and skills of the surgeon and histotechnician
- ▪
Same physician serves as both surgeon and pathologist
- ▪
Precise excision of cancerous tissue
- ▪
Preservation of the maximum amount of non-cancerous tissue
- ▪
Total tissue control and precise mapping
- ▪
Immediate re-excision of residual cancerous tissue as indicated
- ▪
Reconstruction typically performed by Mohs surgeon
- ▪
Exceedingly low complication rate
Introduction
Mohs micrographic surgery (MMS) is a specialized method for the removal of skin cancer that combines surgery with pathology. It has an evidence-based, 5-year cure rate of 99% for basal cell carcinoma (BCC) and 94% for cutaneous squamous cell carcinoma (SCC) . The goals are to remove only the cancerous tissue while viewing the complete margin, thereby preserving the maximum amount of normal tissue (i.e. tissue-sparing) and providing margin control. From a functional, aesthetic and reconstructive perspective, tissue-sparing is especially important for skin cancers in the following areas – periorbital, nasal, auricular, perioral, digital, and anogenital. MMS is also the preferred treatment method for skin cancers that are poorly defined clinically, recurrent, and/or have a higher risk of recurrence because of histologic features or anatomic location ( Table 150.1 ).
| INDICATIONS FOR MOHS MICROGRAPHIC SURGERY FOR NON-MELANOMA SKIN CANCER |
|---|
| Tumor characteristics |
|
| Characteristics of background skin |
|
| Patient characteristics |
|
** High degree of nuclear polymorphism, high mitotic rate, or low degree of keratinization.
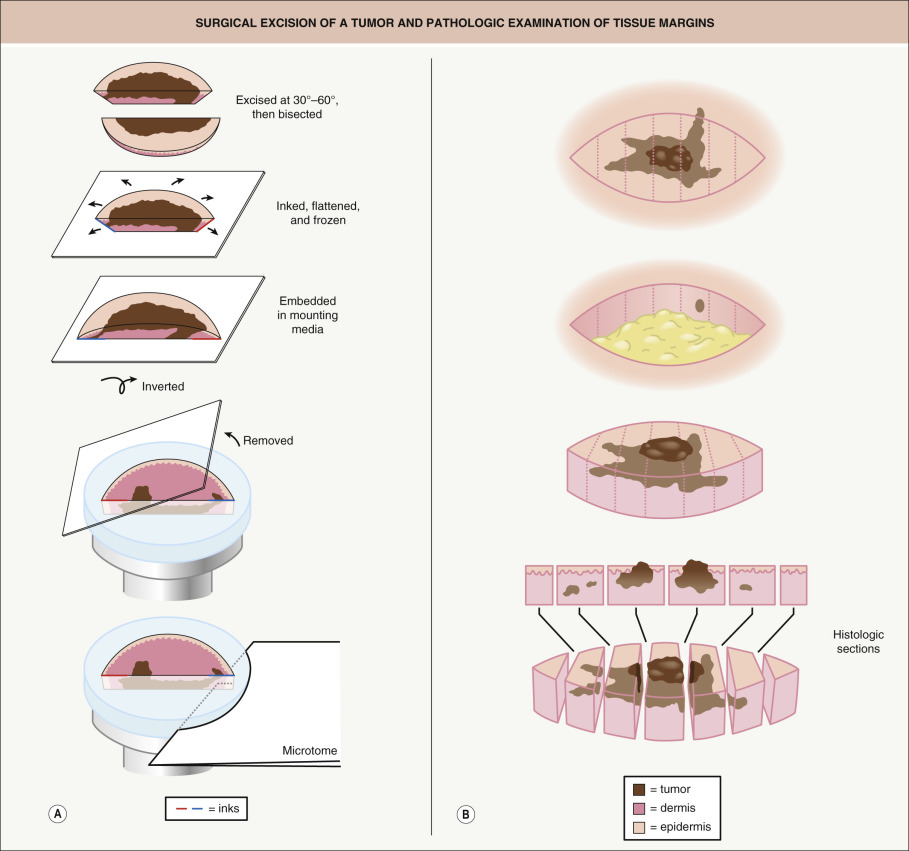
There are four major components: surgical excision, histopathologic examination, precise mapping, and wound management. The surgical component consists of removing a disc of tissue with a beveled surgical margin ( Fig. 150.1A ). The disc of tissue, or “layer”, ensures that the entire 360° of tumor margin is circumferentially removed and examined. A beveled plane of excision allows the tissue specimen sides and bottom to be flattened completely into a single plane. The histopathologic component involves removing several thin sections from the bottom of the flattened cryopreserved tissue specimen and mounting them onto glass slides, followed by staining and microscopic examination for residual tumor. This processing technique differs from standard excisions in which specimens are formalin-fixed, paraffin-embedded (FFPE) and cut in a “bread-loaf” fashion ( Fig. 150.1B ). Precise mapping entails marking any residual tumor on the map or “graph” of the excision with the remaining positive area of tumor re-excised as indicated. This three-step process is then repeated until there is no residual tumor identified microscopically. During this procedure, the Mohs surgeon has complete control of the excised tissue, including laboratory processing and interpretation of the tissue sections, as well as precise mapping. In the fourth step, wound management , the tumor-free defect is surgically closed or allowed to heal by second intention.
History
Originally termed chemosurgery , MMS was developed by Dr Frederic E Mohs, whose pioneering work was performed at the University of Wisconsin-Madison and first published in 1941 . His original clinical technique involved the application of a zinc chloride paste to cancerous tissue 24 hours prior to surgical removal of the tumor. The paste fixed and isolated the tumor in vivo . Subsequently, the tumor was excised and processed in a manner similar to the technique used today. However, if there were residual tumor at the margins, zinc chloride paste was re-applied to cancerous margins for 24 hours prior to any further tissue resection. These steps were repeated until complete tumor clearance was obtained .
In addition to the long incubation period, a second issue is that zinc chloride paste causes tissue necrosis, rendering wounds unsuitable for surgical reconstruction. From this limitation, Dr Mohs’ second major innovation emerged – an objective analysis of second intention wound healing. Historically, surgical dogma held that second intention healing produced inferior cosmetic results compared to reconstruction, the surgeon’s “first intention for healing”. The acceptable, and at times superior, cosmetic outcome associated with second intention healing, particularly on concave surfaces, was documented by Dr Mohs, affording second intention healing its rightful place among valid reconstructive techniques.
Over 40 years ago, the zinc chloride-based, fixed tissue technique evolved into the fresh-frozen tissue technique , which was popularized by Tromovitch and Stegman . The fresh-frozen section technique eliminated the need for the escharotic zinc chloride paste, and it allowed complete tumor clearance via multiple resections in a single day. Furthermore, without a zinc chloride-induced necrotic base, immediate surgical reconstruction could be performed. The fresh tissue technique has now become the standard protocol for MMS.
Training in Mohs Micrographic Surgery
Training in MMS involves all aspects of cutaneous oncology, tumor resection, tissue preparation, histologic interpretation, and reconstruction. The substantial clinical, surgical and pathologic training received during a dermatology residency provides the background for specialized training in MMS. One of the unique advantages of Mohs surgical training is the degree of expertise in clinicopathologic correlation, which derives from dual training in both clinical and pathologic tumor assessment. The dermatologic surgeon must also acquire a mastery of cutaneous and soft tissue anatomy as well as reconstruction, with an emphasis on both function and aesthetic restoration. MMS training programs differ depending upon the country, with a one-year fellowship available in the US .
Indications
Most skin cancers are locally invasive and, depending on their location, they can invade vital structures such as the eye, nose, lips and ears, leading to significant destruction. With time and neglect, metastasis may develop. However, the majority of non-melanoma skin cancers are low-risk and do not fulfill the criteria outlined in Table 150.1 . For such tumors, cost-efficient superficial ablative techniques (e.g. electrodesiccation and curettage) or simple excision can be performed. Currently, ~25% of skin cancers are treated with MMS in the US . As previously discussed, MMS is well suited for skin cancers at high risk of recurrence and for those tumors located in regions requiring tissue conservation, as well as when complete margin control is necessary .
The definition of “high risk” for skin cancer depends on multiple factors, including the clinical and histologic characteristics of the tumor, the anatomic site, and a history of prior treatments (see Table 150.1 ). Because of the complete histologic examination of surgical margins, MMS allows tracking of unpredictable superficial or deep projections of tumor (see Fig. 150.1A ), and it is very useful for tracking tumors that involve embryonic fusion planes, the periorbital region, nerves, and bone. It is these deep and irregular tumor extensions that are primarily responsible for the higher recurrence rates with other techniques. The use of MMS for each of the more commonly managed cutaneous malignancies will be reviewed ( Table 150.2 ). Importantly, the indications for MMS are not absolute, and any treatment decision needs to be made in collaboration with the patient’s preferences, performance status, and understanding of other available treatment options and associated cure rates.
| CUTANEOUS TUMORS TREATED WITH MOHS MICROGRAPHIC SURGERY AND REPORTED CURE RATES | |
|---|---|
| Cutaneous tumors | Cure rates (5-year) |
| Basal cell carcinoma | 99% (primary tumors) 90–93% (recurrent tumors) |
| Squamous cell carcinoma | 92–99% (primary tumors) 90% (locally recurrent tumors) |
| Erythroplasia of Queyrat | 90% |
| Keratoacanthoma | 97.5% * |
| Cutaneous melanoma in situ , including lentigo maligna | 98% |
| Atypical fibroxanthoma | 93–100% |
| Dermatofibrosarcoma protuberans | 97–100% |
| Merkel cell carcinoma | 84% |
| Microcystic adnexal carcinoma | 90% |
| Sebaceous carcinoma | 89% |
| Extramammary Paget disease | 92% |
| Leiomyosarcoma (superficial) | 87% |
Appropriate Use Criteria
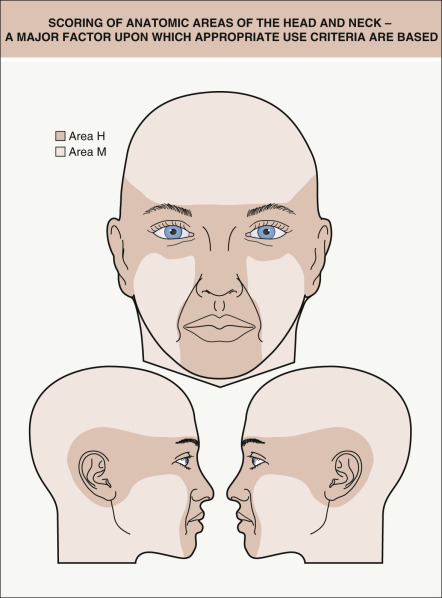
In 2012, there was a multi-society collaboration to develop appropriate use criteria (AUC) for MMS . A panel of Mohs surgeons and medical dermatologists analyzed 270 clinical scenarios in which the tumor locations ( Fig. 150.2 ), sizes, and histologic features varied as did whether or not the tumor was recurrent. The purpose of the Mohs surgery AUC was to provide guidance in the rational use of MMS and to identify tumors (e.g. actinic keratoses, superficial BCCs on the trunk) that did not require MMS treatment . The Mohs surgery AUC are available via a dedicated website ( www.aad.org/education/appropriate-use-criteria/mohs-surgery-auc ) as well as the Mohs AUC app. Additionally, one can refer to NCCN guidelines for the treatment of cutaneous malignancies .
Cost-Effective Care
With the escalating cost of medical care, it is important to be attentive to the appropriate use of therapies and knowledgeable regarding the evidence upon which they are based. The studies on the cost effectiveness of MMS have produced variable results and conclusions, depending on the study location and methods . However, application of the Mohs surgery AUC should lead to greater cost-effectiveness. When standard excision with FFPE sections and immediate repair is compared with MMS, the cost differential is comparable or slightly less expensive with the former (standard excision with immediate repair). However, MMS is considered a cost-effective skin cancer treatment when the following factors are taken into account: the physical location of the surgical procedure (office-based versus ambulatory surgery center), the manner in which the specimen is processed (frozen versus permanent), the type of reconstruction (primary/second intention versus flap/graft) based on the size of the defect due to tissue-sparing versus wide local excision, and the need for additional surgery for tumor recurrences .
Basal Cell Carcinoma
For primary BCCs, cure rates of 87% to 95% can be obtained with superficial ablative therapies and excision, especially on the trunk and extremities. In the meta-analysis by Rowe et al. , the 5-year cure rate for primary BCC treated with MMS was 99%, as compared to 90% to 93% using standard treatments ( Table 150.3 ). For recurrent BCC treated with MMS, the 5-year cure rate was 90–94% as compared to 80% for standard treatments (surgical excision, radiotherapy, cryotherapy, electrodesiccation and curettage) .
| FIVE-YEAR CURE RATES FOR PRIMARY BCC AND SCC | ||
|---|---|---|
| Treatment modality | 5-year cure rate | |
| BCC (%) | SCC (%) | |
| Surgical excision | 90 | 92 |
| Electrodesiccation & curettage | 92 | 96 |
| Radiation | 91 | 90 |
| Cryotherapy | 93 | N/A |
| All non-Mohs modalities | 91 | 92 |
| Mohs micrographic surgery | 99 | 97 |
In addition, MMS provides superior cure rates for high-risk BCCs, including those that are large in size (>2 cm) or have aggressive histologic subtypes or perineural invasion . For example, large BCCs have generally been present for long periods of time and are at greater risk for recurrence, especially if treated with standard methods. When large lesions are present in cosmetically important areas, the sparing of normal tissue is also a critical advantage of MMS. Lastly, basosquamous cell carcinoma, a hybrid between basal and squamous cell carcinoma, has a high risk of local recurrence and is also well managed with MMS .
Squamous Cell Carcinoma
When compared with other treatments, MMS offers the highest cure rates for patients with high-risk primary or recurrent SCCs. The local recurrence rates, based on a retrospective review of MMS versus non-MMS modalities for primary SCC, are outlined in Table 150.3 .
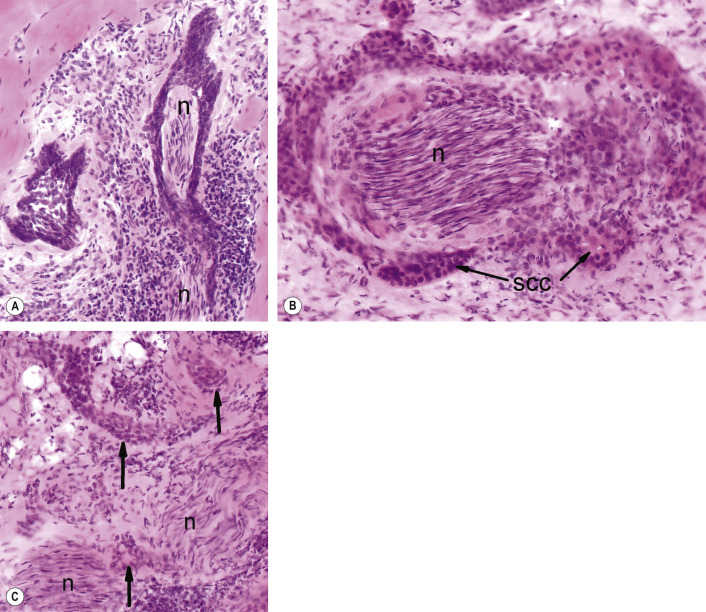
MMS is indicated for management of SCCs with one or more risk factors for recurrence or metastasis (see Table 150.1 ). These indications include large SCCs (>2 cm), recurrent tumors, incompletely excised SCCs, and tumors with ill-defined clinical margins, rapid growth, an aggressive histologic subtype, and/or perineural invasion ( Fig. 150.3 ), as well as SCCs arising in chronic scar tissue . The latter, termed Marjolin ulcers, can behave aggressively, and a multidisciplinary approach with regional node assessment and reconstruction may be necessary in these patients. Numerous, rapidly arising SCCs may affect immunosuppressed solid organ and hematopoietic stem cell transplant recipients or individuals with chronic lymphocytic leukemia, and MMS is well suited for the management of these neoplasms.
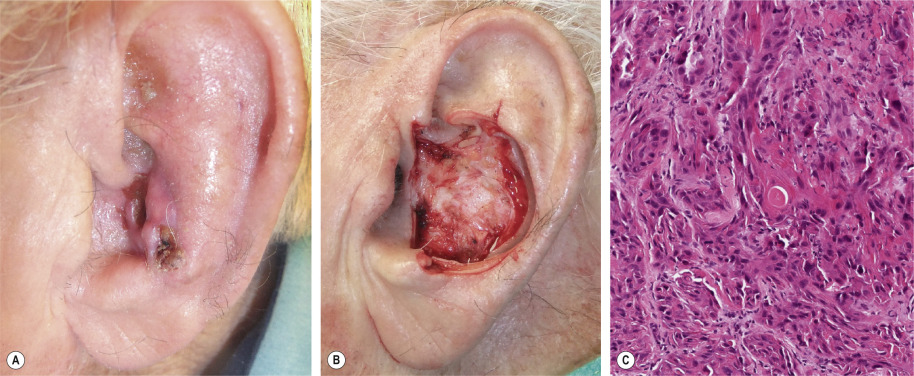
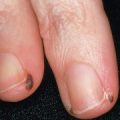
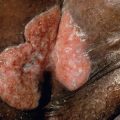
In addition, SCC arising in high-risk anatomic sites may be managed with MMS. For example, tumors of the lip have a high propensity for recurrence and metastasis, and cure rates as high as 92% have been obtained with MMS . MMS is also well suited for SCCs occurring in anogenital areas and on the digits, where tissue conservation is important. When compared with conventional methods, MMS for the treatment of penile SCC offers comparable survival rates. Prior to MMS, SCCs involving the nail unit were often treated with amputation or radiation. MMS provides an excellent choice for treatment of periungual and subungual SCC without osseous involvement, with cure rates of up to 96% .
Erythroplasia of Queyrat
Erythroplasia of Queyrat (EQ) is a term for SCC in situ confined to mucosal epithelium. In men, an erythematous thin plaque is typically seen on the inner surface of the foreskin, glans penis, and/or coronal sulcus (see Chs 73 & 108 ). Of note, EQ is seen primarily in uncircumcised men. In women, mucosal SCC in situ favors the labia minora and the vestibule. Oncogenic human papillomaviruses often play an etiologic role, and immunosuppressed individuals (e.g. HIV-infected) are at greater risk for developing anogenital SCC in situ .
MMS is an effective treatment for EQ, with a 5-year cure rate of 90% . When excising EQ of the glans penis, Mohs layers should be very thin in order to minimize entry into and resection of the corpus spongiosum and cavernosum. If either is entered, achieving hemostasis can be challenging. EQ can often extend into the urethra and differentiation of SCC in situ from transitional epithelium is often difficult on frozen sections. With deep extension along the urethra, collaboration with a urologist and completion of the surgery under general anesthesia are recommended.
Keratoacanthoma
Keratoacanthoma (KA) may be considered a well-differentiated SCC with specific histologic features. KAs classically grow quickly and then spontaneously involute; however, there are exceptions and metastasis has been reported, implying that the tumor was actually an SCC. Also, microscopically, some KAs may be difficult to distinguish from an SCC. As a result, nowadays, most KAs are considered to be well-differentiated SCCs and are managed with excisional surgery or MMS. MMS may be particularly beneficial for large lesions, as well as those occurring in anatomically sensitive areas. In one study, a 2-year cure rate of 97.5% was observed for KAs treated via MMS (see Table 150.2 ) .
Verrucous Carcinoma
Verrucous carcinoma is considered a variant of SCC, and it can be triggered by the human papillomavirus. It occurs most commonly in the oral cavity, on the plantar surface of the foot, or on the penis (see Ch. 108 ). While generally considered a low-grade carcinoma, there are reports of metastasis. Because the tumors are usually localized, MMS is an excellent treatment option. Histopathologic interpretation of frozen sections can be challenging because the tumor cells are very well differentiated and lack nuclear atypia, making distinction from epithelial hyperplasia, verruca vulgaris, or condyloma acuminatum difficult.
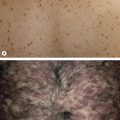
Lentigo Maligna
Since lentigo maligna (melanoma in situ of sun-damaged skin) may be a clinically and histologically ill-defined tumor, it has a high local recurrence rate with standard wide-excisional surgery. Although frozen section histology of lentigo maligna can be challenging, MMS offers the possibility of complete margin examination and tissue sparing. Immunohistochemical (IHC) staining of frozen sections (see below) may improve diagnostic accuracy . Because of the challenge of detecting atypical melanocytes in frozen sections, some surgeons employ techniques such as wide excision Mohs surgery, “slow” Mohs staged excision (utilizing FFPE sections), and geometric staged excision (see Variations) .
Melanoma Other Than Lentigo Maligna
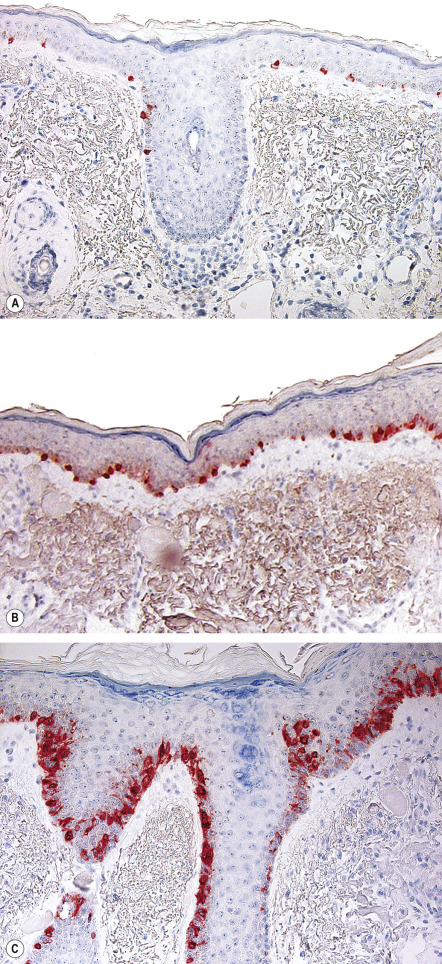
The role of MMS that utilizes only frozen sections for the treatment of cutaneous melanoma is controversial for several reasons. These include: the challenge of detecting atypical melanocytes in frozen sections; the margin taken is often narrower than published guidelines; and the higher risk of melanomas to metastasize. The use of special stains, such as Melan-A/MART-1, HMB45, Mel-5, and S100, can increase histologic sensitivity during MMS for melanocytic lesions ( Table 150.4 ) ; however, because some of these stains target melanosomal proteins, there is also staining of keratinocytes. For this reason, use of melanocyte-specific nuclear stains, e.g. MITF, may prove useful. Even so, some dermatologic surgeons advocate the examination of FFPE tissue margins to confirm that the final MMS margin is negative .
| SPECIAL AND IMMUNOHISTOCHEMICAL STAINS USED IN MOHS MICROGRAPHIC SURGERY | |||||||
|---|---|---|---|---|---|---|---|
| SCC * | BCC | DFSP | EMPD | Sebaceous carcinoma | Melanoma ** | MAC | |
| Special stains | – | Toluidine blue | – | PAS | Oil red O § | – | Toluidine blue |
| Immunohistochemical stains | AE1/AE3 (inset)  | AE1/AE3, Ber-EP4 | CD34 | CK7, CEA | Adipophilin, CK (e.g. AE1/AE3) | MITF, Melan-A/MART-1 (see Fig. 150.6 ), Mel-5, SOX10, HMB45 | CK (e.g. AE1/AE3) |
* To detect perineural invasion and high-risk histologic subtypes, e.g. infiltrating, spindle cell, single cell.
** Includes lentigo maligna and other types of melanoma in situ .
Although uncontrolled, the extensive experience of Zitelli and colleagues in the management of melanoma with MMS provides reassurance that outcomes are equivalent to standard margins . While many clinicians do not find significant benefits to MMS for routine management of melanoma (e.g. most tumors are well defined), specific clinical scenarios appear especially appropriate: locally recurrent melanoma; tumors with a large diameter; ill-defined or amelanotic melanoma; and melanoma near critical anatomic structures such as the genitals, digits, eyelids, nose, and ears. Surgeons can opt to use the techniques listed previously for lentigo maligna which employ histologic principles similar to those of FFPE-based pathology in order to attempt to achieve complete margin control (see Variations) .
Atypical Fibroxanthoma
Atypical fibroxanthoma (AFX) typically presents as an ulcerated nodule in the head and neck region of an elderly person with sun-damaged skin. In general, it grows in a contiguous fashion. Histologically, AFX is composed of spindle cells mixed with bizarre, multinucleated giant cells, making the tumor relatively easy to identify on frozen sections. MMS has demonstrated utility in the management of AFX, especially in areas of cosmetic importance .
Dermatofibrosarcoma Protuberans
Dermatofibrosarcoma protuberans (DFSP) is an uncommon, slow-growing, locally aggressive soft tissue sarcoma that most often arises on the trunk of young to middle-aged adults. DFSP typically invades beyond the clinically visible tumor margins with unpredictable and irregular extensions. Based upon pooled raw data from the past 20 years, recurrence rates following wide local excision have ranged from 6% to 60% versus ~1% to 3% following MMS . Explanations for this difference include the extensive continuous subclinical expansion of DFSP, the low risk of distant metastasis, the indolent rate of growth, and identifiable histologic features in frozen sections . Removal of DFSP with MMS can be time consuming due to the frequently large size of these tumors. The “slow” Mohs staged excision technique with “rush” FFPE sections can be utilized, especially for those DFSP tumors that are difficult to interpret on frozen sections. CD34 staining of permanent sections can be employed to better define the tumor cells .
Merkel Cell Carcinoma
Merkel cell carcinoma (MCC) is an uncommon, but very aggressive, skin cancer, usually arising in elderly patients (see Ch. 115 ) . There is usually a history of significant cumulative sun exposure or immunosuppression. Clinically, the tumor presents as a pearly to red–blue papulonodule and is often in the head and neck region. Microscopically, the tumor cells are uniformly deep blue, polygonal in shape and aggregate in large sheets, allowing them to be easily identified in frozen sections. Treatment consists of complete surgical removal of the primary tumor via either MMS or wide excision plus sentinel lymph node biopsy , followed by radiotherapy to the primary site and regional lymph nodes (unless the lesion is low risk; see Fig. 115.19 ).
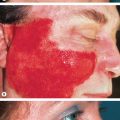
Microcystic Adnexal Carcinoma
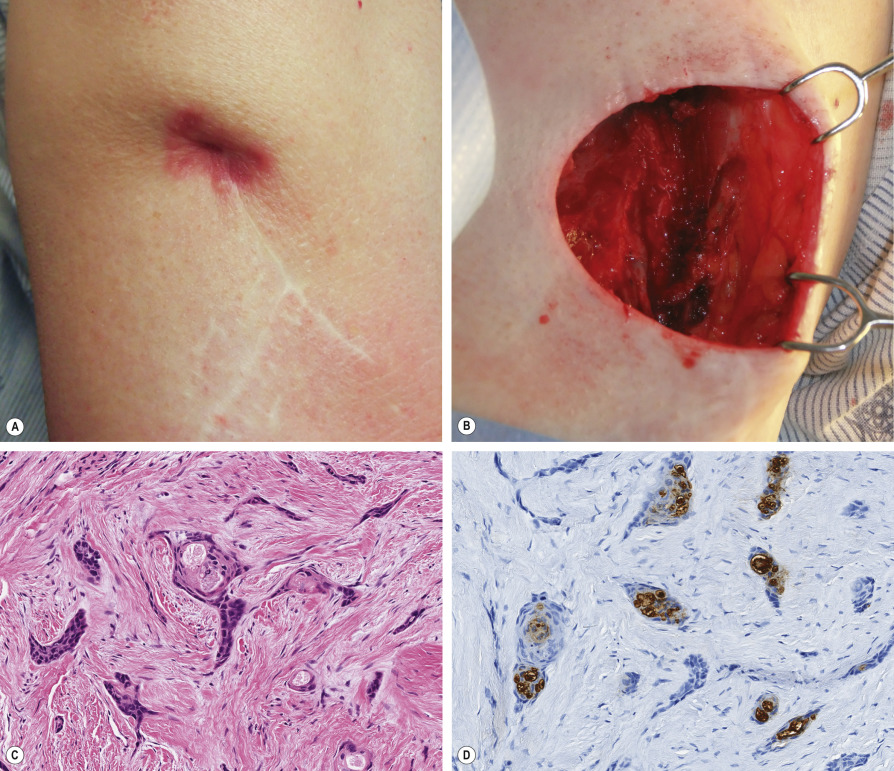
Microcystic adnexal carcinoma (MAC) is an uncommon, locally aggressive tumor that typically involves the face of older adults ( Fig. 150.4 ). Histologically, it has an infiltrative growth pattern with frequent perineural invasion ( Fig. 150.5 ). In the literature, the overall recurrence rate for MAC approaches 40%. To date, MMS appears to offer the highest cure rate (see Table 150.2 ) . A study by Chiller et al. demonstrated that defects following MMS were four times larger than clinical estimates. They concluded that preoperative estimation of margins is generally unreliable, and complete histologic verification of clear margins is advisable.
Sebaceous Carcinoma
The use of MMS for management of sebaceous carcinoma has also been debated, primarily due to reports of discontiguous growth, which can thwart any method of histologic margin evaluation. The desire for tissue conservation on the eyelid (most common site) makes MMS a logical approach. The removal of an extra safety margin of tissue for permanent sections may be a reasonable strategy with this type of tumor. In a recent literature review, wide local excision of sebaceous carcinomas was associated with a local recurrence rate of 4–37% and a nodal metastatic rate of 3–28% . In comparison, MMS has a reported local recurrence rate of 11–12% and a regional metastatic rate of 6–8% . However, in a recent retrospective review of 45 tumors treated with MMS, there were no local recurrences, metastases, or disease-specific deaths, with an average follow-up of 3.6 years .
Other Adnexal Carcinomas
This group consists of the uncommon tumors that have sweat gland (eccrine, apocrine), sebaceous gland, or follicular differentiation and includes mucinous eccrine carcinoma, eccrine porocarcinoma, adenoid cystic carcinoma, papillary eccrine carcinoma, and extramammary Paget disease (EMPD). Except for EMPD of the groin or sebaceous carcinoma of the eyelid, the clinical features are rarely diagnostic . As a group, these tumors are locally destructive with extensive unpredictable local tissue invasion, have a high rate of local recurrence when treated by wide excision, and metastasize late in the course of the disease. Typically, their microscopic features are readily recognizable in frozen sections so they are amenable to MMS. Rapid immunohistochemistry can aid in diagnosis (see Table 150.4 ). IHC staining of FFPE sections can also serve to confirm the initial diagnosis as well as margin clearance.
Other Tumors and Applications
MMS has been used to treat a number of other malignant neoplasms, including angiosarcoma, lymphoepithelioma-like carcinoma, leiomyosarcoma, and cutaneous breast metastases , as well as benign tumors that tend to recur, such as infantile digital fibroma, desmoplastic trichoepithelioma, and granular cell tumor. The complete margin examination of MMS may provide enhanced tumor clearance. MMS has also been used to extirpate difficult-to-treat cutaneous fungal infections.
Contraindications
Discontiguous tumors can create problems with false-negative margins, but this problem occurs with all other methods employed to evaluate surgical margins histologically. Therefore, it is not an absolute contraindication. If a tumor cannot reliably be identified histologically, especially on frozen sections, this can be a relative contraindication. For example, lentigo maligna can sometimes be difficult to distinguish from the proliferation of atypical melanocytes found in adjacent sun-damaged skin uninvolved by malignancy ( Fig. 150.6 ). If patients are unwilling or unable to undergo surgery that is time intensive, then standard excision or a non-surgical approach such as radiotherapy may be a better option. However, for some patients with serious medical problems, MMS done under local anesthesia may be safer than surgery performed under general anesthesia .