Chapter 30 Modulation of the hypermetabolic response after burn injury
![]() Access the complete reference list online at http://www.expertconsult.com
Access the complete reference list online at http://www.expertconsult.com
Introduction
Treatment of the sequelae induced by a severe burn injury presents a substantial medical management challenge. More than 1 million burns occur in the USA annually. Even though the majority of these patients have minor injuries, nearly 20% of patients with thermal injuries are admitted for treatment at specialized burn centers each year.1 Advances in clinical knowledge and treatment strategies have resulted in a major decline in burn-associated deaths over the last two decades; these advances include defining the hypermetabolic response, refining reconstructive techniques, supporting inhalation injury in an aggressive manner, and improving infection control.2,3
Despite these improvements in clinical care, severe burn injuries can still lead to multi-system organ failure, with associated morbidity and mortality.4 The dramatic metabolic changes underlying severe burn injury clearly contribute to unfavorable outcomes in severely burned patients.4,5
Metabolic alterations following severe burn injury
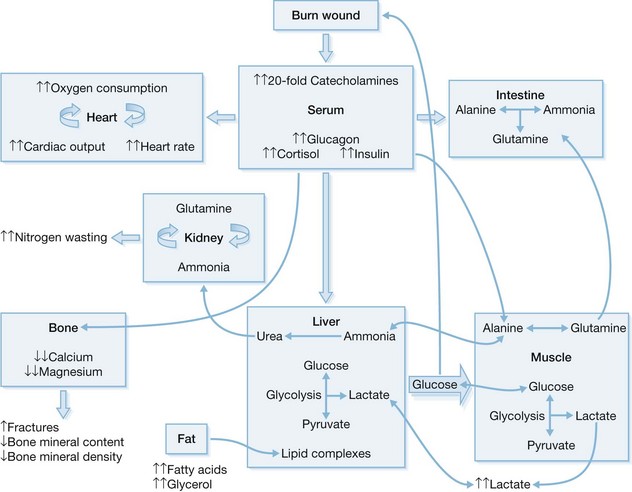
Characterization of the response to severe burn injuries covering over 40% of the total body surface area (TBSA) has shown the association of severe hypermetabolism and inflammatory and stress responses with development of a hyperdynamic circulation, altered body temperature regulation, glycolysis, proteolysis, lipolysis, and inefficient substrate cycling.6–8 The severity, duration, and magnitude of the metabolic changes are uniquely different in burn patients than in other critically ill patients.4 Enhanced secretion of catecholamines, glucocorticoids, glucagon, and dopamine is closely associated with the formation of the acute hypermetabolic response and the associated catabolic state.6,9–16
However, the underlying mechanisms of this complex response to burn injury remain unclear. Based on current knowledge, a variety of cytokines (including tumor necrosis factor (TNF), platelet-activating factor (PAF), interleukin (IL)-1, and IL-6), endotoxin, nitric oxide, reactive oxygen species, and complement cascades participate in this multifactorial response to burn injury.17 These modulators act to increase metabolic rate and alter glucose metabolism post-burn.18
The metabolic derangements after burn injury occur in two distinct patterns that are time-dependent.19 Within 48 h post-burn, decreases in oxygen consumption, cardiac output, metabolic rate, and glucose tolerance are considered the “ebb phase.”19,20 Amplification of these metabolic changes within the first 5 days post-burn to a plateau characterizes the second phase. This so-called “flow phase” is typically characterized by the development of a hyperdynamic circulation alongside the hypermetabolic state.
Multiorgan dysfunction results from the extreme activation of the acute phase response post-burn. Immediately post-burn, patients typically have low cardiac outputs characteristic of early shock.21 However, 72–96 h post-burn, at the onset of shock, cardiac outputs are >150% of those in non-burned, healthy volunteers.22 Heart rates in burn patients approach 160% of those in non-burned, healthy patients.23 After severe burn injury, cardiac work is significantly elevated, and this lasts well into the rehabilitation phase.4,24 Myocardial oxygen consumption values far surpass values seen in trained long-distance runners, and these values remain elevated far into the rehabilitative phase.25 A profound hepatomegaly also develops after burn injury. We have shown that the liver may increase by 225% within 2 weeks of injury and remains enlarged at discharge by 200%.22
The release of insulin during this time period is increased in response to glucose load when compared with controls,24,26 and glucose levels remain significantly elevated, signifying an insulin-resistant state.26,27 Although it was believed that these metabolic derangements resolved as the wound healed, we and others have demonstrated that the duration of this hypermetabolic response may exceed 1 year after burn trauma.6,9,16,28 Post-burn hypermetabolic alterations may last for up to 36 months and include elevations in cortisol, cytokines, and catecholamines; increased resting energy expenditures; hindered glucose metabolism; and decreased insulin sensitivity.
In healthy human beings, a postprandial increase in serum glucose levels stimulates insulin secretion from pancreatic β-cells, which in turn decreases hepatic gluconeogenesis and supports peripheral uptake of glucose into adipose tissue and skeletal muscle.29,30 Acutely after burn injury, profound derangements in energy substrate metabolism occur. The anabolic properties of insulin are inhibited by stress mediators (cortisol and catecholamines) to increase delivery of glucose to vital organs.31 In thermally injured patients, the availability of gluconeogenic substrates is increased by lipolysis of adipose tissue32 and proteolysis of skeletal muscle.33 This, in turn, augments hepatic glucose production (Fig. 30.1).29,30,34,35 The normal feedback mechanisms are distorted as part of the response to burn. Elevated blood glucose levels fail to suppress hepatic glucose release,36 while the inhibitory effects of insulin on hepatic glucose secretion exacerbate hyperglycemia after severe burn injury.37 This is further complicated by catecholamine-mediated glycogenolysis in the liver and sympathetic stimulation of glycogen breakdown.30 Catecholamines impair glucose disposal and cause peripheral insulin resistance by altering the insulin signaling pathway and by changing GLUT-4 translocation in adipose and muscle tissue (Fig. 30.1).29,38 In fact, in muscle biopsied at 1 week post-burn, insulin receptor signaling via Akt was diminished.37 A physiologic link exists between impaired mitochondrial oxidative function in the liver versus the muscle, resulting in altered lipolysis and impaired insulin signaling post-burn. This affects the inhibitory actions of insulin on glucose production in the liver and alters glucose uptake into skeletal muscle.26,32,36,37
Glucagon plays a major role during the acute phase post-burn as well. Like epinephrine, glucagon increases the production of glucose by glycogenolysis and gluconeogenesis.39 These actions are further intensified by secretion of various proinflammatory cytokines.40–42 Specifically, IL-6, TNF, and monocyte chemotactic protein (MCP)-1 act directly on the insulin signal transduction pathway through insulin receptor substrates, resulting in both post-burn hyperglycemia insulin resistance43–45 and lean muscle catabolism during the acute and convalescent phases post-burn.46,47
During starvation, energy is supplied by lipolysis and ketosis. Marked lean body mass (LBM) wasting occurs because the burn patient preferentially uses skeletal muscle as the major source of fuel.4,48 Nitrogen balance studies (whole body and cross leg) show persistent muscle breakdown for up to 9 months post-burn.49 Importantly, as the skeletal muscle accounts for the majority of insulin-stimulated, whole-body glucose uptake, significant LBM loss may contribute to post-burn insulin resistance.50 Flakoll and co-workers51 studied the relationship between muscle protein catabolism and hyperglycemia, demonstrating that a significant increase in proteolysis rates was not accompanied by changes in leucine oxidation or non-oxidative disposal. They also demonstrated that increased plasma glucose levels stimulate whole-body proteolysis during hyperinsulinemia.51 Catabolic losses in LBM correlate with increased morbidity and mortality: marked delays in wound healing and significant increases in infection rates accompany a loss of 10% of LBM;52 wound healing is impaired following a 20% loss in LBM; severe infections are associated with losses of 30% of LBM; losses of 40% or more ultimately lead to death. Acutely, the net LBM losses from muscle wasting lead to prolonged mechanical ventilation, inhibition of cough reflexes, and a delay in mobilization, markedly contributing to increased mortality in these patients.53 Chronically, these net losses reduce strength and the possibility for full rehabilitation. Our patients experience an average nitrogen loss of 20–25 g/m2 TBSA/day, a rate at which lethal cachexia becomes imminent in less than 1 month if left untreated. Continued post-burn loss may persist for up to 9 months. Persistent protein catabolism probably accounts for the growth delay that frequently occurs in our pediatric burn patients.54
Non-pharmacologic approaches that ameliorate the post-burn hypermetabolic response
Early wound closure
In recent years, burn wound management has changed, and the most significant advances in burn care have been the early excision of necrotic tissue and burn wound closure. This has significantly diminished basal energy requirements and in turn, improved mortality rates.55–59 Early closure of the burn wound is also associated with diminished incidence of excessive scars and joint contractures and is responsible for faster rehabilitation of these patients.55,58
Currently, powered dermatomes or hand skingraft knives are used to excise most burn wounds. Excisions via electrocautery or knife are usually used in areas where optimal function or cosmesis are important. Preservation of viable dermis may be possible in partial thickness wounds. However, excision of all necrotic and infected tissue is paramount in full-thickness burn wounds.60 Tangential, full-thickness, and fascial excision techniques are typically used. During tangential excision, the deep dermal partial thickness burn is repeatedly shaved using a knife (Goulian, Watson, or Braithwaite) or a dermatome (set to a depth of 5–10/1000 inch). Necrotic or infected tissue is shaved until punctate dermal wound bed bleeding signifies the uncovering of the viable dermal wound bed.60 Full-thickness excision and serial passes are made to excise the full-thickness wound using a Watson or powered dermatome (set at 15–30/100 inch). In this case, the complete excision is indicated by reaching an actively bleeding wound bed.60 Fascial excision is the surgical removal of subcutaneous fat until the fascia is reached. It is used for burns that extend deep into the muscle or when invasive fungal infections are present. However, this technique generally leaves a permanent contour defect.60
During these operations, blood loss is a critical issue, as approximately 5% of the total blood volume is lost with the excision of every 1% of the body surface.61,62 Blood loss is a major determinant of morbidity and mortality,63 and a variety of techniques need to be used to control bleeding, including the local application of fibrin or thrombin sprays, epinephrine-soaked pads (1 : 40 000), topically administered epinephrine (1 : 10 000–1 : 20 000) or electrocautery of blood vessels.64 Additional use of tourniquets for pre-excisional tumescence with epinephrine and saline may also help limit blood loss.65
Nutritional support
Patients suffering from severe burns who are treated solely with vigorous oral supplementation may lose one-quarter of their pre-admission weight within the first several weeks.66 Therefore, adequate nutrition is a critical issue for severely burned patients. A high-calorie nutritional intake is associated with diminished muscle metabolism after burn.67 However, high-calorie feeding has recently been associated with enhanced mortality rates.68 Instead of overfeeding, adequate caloric enteral intake is currently recommended.4,58 Various formulations have been established to adequately meet the energy requirements of each individual burn patient.69–71 The Curreri formula (25 kcal/kg per day plus 40 kcal/% TBSA burned per day) is normally used to calculate the caloric requirements in adult burn patients.72 The marked post-burn changes in lipid, carbohydrate, and protein metabolism determine the caloric needs of each respective patient. The optimal dietary composition includes 1–2 g/kg per day of protein, supporting the synthetic needs of the patient, thus counteracting the proteolysis occurring in muscle tissue.48 Many intensive care units (ICUs) deliver a substantial percentage of calories as fat because essential fatty acid deficiency frequently accompanies long-term nutritional supplementation.73 This approach reduces carbohydrate requirements and burn-induced glucose intolerance, and therefore, dietary compositions of 30–50% fat are now the standard of care when treating critically ill patients.48 Unfortunately, enhanced fat administration in burn patients is associated with hepatic steatosis, hypoxemia, hyperlipidemia, higher infection rates, and higher postoperative mortality rates.74–76 Hepatic triglyceride levels are enhanced, thereby limiting the utility of exogenous lipids as a post-burn energy source.32,73,77,78 Recent data obtained by our group demonstrated that patients receiving Vivonex® T.E.N., a low-fat/high carbohydrate diet, had a significantly lower incidence of hepatic steatosis than milk-fed burn patients. We also demonstrated improved survival, significantly lower incidences of sepsis, reduced length of ICU stay per % TBSA, and markedly decreased average ICU stay. We thus advocate nutritional regimens for burn patients that include reduced proportions of fat.
Environmental support
Burn patients may lose a significant portion of body water (approximately 4000 mL/m2 burned/day) by way of evaporative or other losses from extensive burn wounds.79 Skin and core temperatures are increased by 2°C above normal to abate the resultant hypermetabolic and catabolic responses by generating a portion of energy needed to offset the losses in heat accompanying the water loss. Increasing the ambient temperature to 33°C may result in a decrease to 1.4 times the resting energy expenditure instead of 2.0 times the resting energy expenditure. This environmental modulation is a key primary treatment goal that is too often under-utilized.80
Exercise and adjunctive measures
Burn-wound contracture remains a morbid consequence of severe burns. It is well established that physical therapy is paramount to preclude burn wound contractures. Progressive resistance exercises maintain or even increase LBM, facilitate the formation of muscle proteins by incorporating amino acids, enhance muscle strength, and increase walking distances by 50%.81 In burned children, progressive resistance exercise can be performed safely without any exercise-related hyperpyrexia. Judicious use of sedation, narcotics support, and supportive psychotherapy are obligatory to reduce long-term morbidity post-burn.82
Pharmacologic approaches to attenuate the metabolic alterations post-burn
Recombinant human growth hormone (rhGH)
As demonstrated by our group, during the acute hospitalization period, daily intramuscular injection of rhGH (0.2 mg/kg) positively alters the acute phase response in the liver,83,84 improves muscle protein kinetics, maintains muscular growth,85,86 improves resting energy expenditure, decreases cardiac output,87 and decreases donor site healing by approximately 1.5 days.88 RhGH mediates its effects through its secondary mediator IGF-I.89 In patients receiving rhGH, serum IGF-I and IGF-binding protein (IGFBP)-3 increase by 100% over levels seen in healthy controls.90,91 Nevertheless, as demonstrated in a recent study by Takala and colleagues, in 532 non-burned critically ill patients, increased rhGH (0.10 ± 0.02 mg/kg) correlated with increased morbidity and mortality rates91 and was associated with hyperglycemia and insulin resistance.92,93 This may be reflective of an age-specific effect, however, as in severely burned children, mortality rates were not affected by either short- or long-term rhGH administration.87,94
Insulin-like growth factor (IGF)
Since the positive effects of rhGH on the post-burn hypermetabolic response are predominantly mediated by the secondary mediator IGF-I, it is not surprising that, in these same patient populations, administration of equimolar amounts of recombinant human IGF-1 with its binding protein, IGFBP-3, successfully enhanced protein metabolism,95,96 attenuated muscle catabolism, restored gut mucosal integrity,96 enhanced immune function, and returned serum concentrations of constitutive proteins to non-burned levels.96–99 However, Van den Berghe et al.100 could not demonstrate any efficacy in non-burned critically ill patients when using IGF-1 alone.
Anabolic agents: oxandrolone
The use of oxandrolone, an analog of testosterone possessing only 5% of its virilizing androgenic effects, also enhances anabolism of muscle protein by improving the efficiency of protein synthesis.101 Oxandrolone decreases loss of body weight and improves healing of the donor site wound.102 In a large clinical trial by our group, oxandrolone administered at 0.1 mg/kg twice daily reduced length of the acute hospitalization, sustained LBM, and improved liver protein synthesis.103 In conclusion, administration of oxandrolone decreased the sequelae of the burn-induced hypermetabolic response and alterations in LBM and bone mineral content.104 Exercise, however, remains an essential tool for developing strength.105
Propranolol
Beta-adrenergic blockade with propranolol may represent the most efficacious anticatabolic therapy for severe burns. As shown in several studies, the administration of propranolol (titrated to decrease heart rate by 15–20%) diminishes cardiac work25
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree