Chapter 44 The burn problem
A pathologist’s perspective
![]() Access the complete reference list online at http://www.expertconsult.com
Access the complete reference list online at http://www.expertconsult.com
![]() IN THIS CHAPTER
IN THIS CHAPTER ![]() PowerPoint Presentation Online
PowerPoint Presentation Online
Introduction
‘Burns are not a simple injury, but a very complicated disease.’ This statement, dating from 1840, holds true with additional force in 2012.1 Massive destruction of skin tissue and smoke-induced injury to the airways both stimulate complex reactions which are still only partly understood. Malfunction of every organ system frequently complicates the responses of patients to large burns. The nature of this malfunction is often clarified by examination of the body after death. Postmortem examination also may reveal unsuspected infections or adverse effects of therapy. In addition, postmortem examination leads to review of the circumstances of injury and of the causal sequence in which complications occurred, in order to determine the manner of death. Analysis of an entire case from the point of view of pathogenesis often clarifies the nature of the patient’s most significant problems. This chapter systematically reviews some of the observations made at autopsy in patients who have died after burn injury. It also surveys experimental evidence that bears on pathogenetic mechanisms relevant to disease processes seen at autopsy. The observations reviewed here are drawn from our experience of 253 autopsies performed on burned children who died at the Shriners Burns Hospital in Galveston, Texas, from 1971 to the present.
To introduce the sections that follow, the major medical problems that complicate burn injury are summarized here. The degree of disruption of normal physiologic processes after burn injury is extreme. Immediately after burn injury, massive loss of intravascular fluid into the burned tissue begins to occur.2,3 Unless this fluid loss is replaced by the physician very promptly and carefully, serious hypovolemia develops.4–6 Hypovolemia and the resulting reduction of tissue perfusion to levels less than that necessary to maintain cellular homeostasis, which is defined as ischemia, causes necrosis of certain cells, typically those with the greatest oxygen demand within a tissue. The neural and endocrine responses to the traumatic injury may lead to recognizable lesions in the stomach and the heart. Ischemia may lead to necrosis of pancreatic acinar epithelium and acute pancreatitis. Thermal injury to skeletal muscle, or lack of perfusion of muscle, may lead to local exudation of fluid and development of such high pressure in fascial compartments that arterial perfusion is prevented. This ‘compartment syndrome,’ unless relieved by prompt surgical intervention, leads to necrosis of muscle throughout the entire compartment.7 The consequences of massive necrosis of muscle often include secondary injury to the lungs, owing to release of reactive oxygen species, and myoglobinuria with secondary renal damage.8 At the time of injury, patients frequently inhale sufficient carbon monoxide to compromise the oxygen-carrying capacity of the blood. The resultant tissue hypoxia can cause death at the scene, and if the patient survives it can be sufficient to lead to irreversible neuronal injury, cerebral edema and brain death. Hypoxia, sometimes related to carbon monoxide intoxication, also contributes to cardiac and renal injury. In addition, when fires occur in closed spaces, the ‘flashover’ process consumes all available oxygen, so that the patient’s environment may contain too little oxygen to sustain life. Occasionally a burn victim is found without pulse or respiratory effort, probably as a consequence of hypoxia, and is revived by cardiopulmonary resuscitation. In such cases ischemic and hypoxic injury may be profound in multiple organs, and there may be significant ‘ischemia–reperfusion injury’ to the lungs after resuscitation. Patients injured in house fires often suffer injury to the respiratory tract caused by inhalation of toxic products of combustion.9 This smoke inhalation injury stimulates an intense inflammatory reaction, which can lead to obstruction of airways and further tissue injury. This problem is discussed below in the section on the respiratory system. Infection is the next major risk experienced by patients after burn injury. Necrotic skin provides an excellent environment for proliferation of bacteria and fungi, and as long as necrotic tissue remains, the risk of infection remains high. Immunosuppression contributes to this risk, so that burn patients develop serious infections with agents otherwise usually encountered in patients treated with immunosuppressive drugs after transplantation. The mechanisms of this immunosuppression are still under investigation, but include excessive secretion of glucocorticoids and abnormal cytokine signaling. Injury to the intestinal epithelium by hypoxia or ischemia leads to translocation of intestinal bacteria into the portal circulation. Coagulopathy may be a very serious complication of burn injury. It may lead directly to tissue ischemia after vascular thrombosis, or the resultant hemorrhage may lead to secondary hypovolemia. Patients often require transfusion of very large quantities of blood products during their treatment and are subject to all the risks of transfusion.
Disease processes involving multiple organ systems
Hypoxia and ischemia
After ischemia and hypoxia have led to irreversible injury and death of selected cell types or whole segments of organs (infarcts), responses are generated which may lead to further injury of remote organs. Cellular necrosis stimulates an intense acute inflammatory reaction, probably initiated by activation of the complement cascade, degranulation of mast cells, and other processes. This reaction surrounds an infarct, or proceeds throughout a region of tissue injury if the local circulation is sufficient. In the skin, the tissues at the base of the zone of necrosis become acutely inflamed. Monocytes recruited to the regions of injury secrete cytokines in large quantities, and polymorphonuclear neutrophils activate their antibacterial mechanisms. Cytokines have effects throughout the body, the most important probably being TNF-α, and superoxide released by neutrophils has important distant effects. In addition, in endothelial cells injured by hypoxia the enzyme xanthine dehydrogenase is converted to xanthine oxidase, which releases superoxide during degradation of adenosine, which in turn is released by necrotic cells.10,11 Superoxide, released into the circulation by this metabolic process and by neutrophils, can injure the lung by damaging both endothelial and epithelial cells and allowing protein-rich fluid to exude into alveoli. The inflammatory reaction to thermal tissue injury also stimulates an intense influx of neutrophils, which undoubtedly contributes to this injury by releasing superoxide. In experimental models of burn injury, as well as in models of ischemia–reperfusion injury, the lungs have been shown to be injured by these processes.12 Endogenous antioxidants such as glutathione are depleted, and conjugated dienes appear, indicating that cell membrane injury due to lipid peroxidation has occurred in the lung.13
Infection and sepsis
The skin normally provides a highly effective barrier against invasion by infectious agents. Necrotic tissue, in the skin as elsewhere, is ineffective as a barrier to infection and indeed provides an excellent culture medium. Patients who are treated for deep burn injury with traditional debridement and washing for several days generally arrive at this institution with large quantities of multiple microorganisms growing in the necrotic skin. The bacteria appear to proliferate initially in areas which have insufficient circulation to develop a significant inflammatory response. As large numbers of bacteria accumulate, those with high pathogenic capacity invade the adjacent viable tissue, produce further necrosis, and gain access to the circulation. This is the condition of burn wound sepsis, which historically has been the leading cause of death in burn patients. In Linares’ series of 115 autopsies, sepsis was present in 73%, as documented by positive blood culture and demonstration of invasive infection of viable tissue.14,15 When pneumonia was found at autopsy in such a case, the cause of death was classified as burn wound sepsis, with pneumonia as a contributing cause. In 80% of these fatal cases of sepsis the burn wound was the source of the infection. The most important pathogens were Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella pneumoniae, Escherichia coli, Enterobacter, and Candida species. More recently, antibiotic-resistant Acinetobacter species have emerged as a frequent cause of fatal sepsis. Burn wound sepsis is suspected clinically when a burn wound is the site of proliferating microorganisms exceeding 105/g of tissue, and there is histologic evidence of active invasion of subjacent unburned tissue.16 In our institution, the wounds of burn patients, especially the open areas, are routinely sampled for quantitative culture and for histologic study when excision and grafting procedures are done, and whenever clinical examination suggests the possibility of tissue infection. The histologic classification used and its rationale are discussed below under the integumentary system. Once septicemia occurs, there is a generalized reaction which often includes hypotension, tachycardia, increased hyperthermia or hypothermia, and poor perfusion of the intestines and other viscera.17 In the case of Gram-negative bacteria, the endotoxin stimulates monocytes via their CD14 receptors to become activated and set up a cascade of release of pro- and anti-inflammatory mediators which affect all organs and tissues in the body.18–20 Coagulopathy is also an important complication of sepsis.21 In addition, once bacteria have gained entrance to the general circulation, it becomes possible for foci of tissue infection to develop at distant sites. This is most likely to occur in sites of tissue necrosis, on cardiac valves, or in the lungs. Abscesses in distant organs can allow the infection to resist eradication by specific antibiotics, and thus allow sepsis to persist despite appropriate therapy. One recent case demonstrated clearly the route of dissemination of fatal burn wound sepsis. The patient was admitted in a clinically septic condition 2 weeks after suffering a large burn, and died in spite of aggressive therapy. At the time of autopsy there were many areas of bacterial proliferation within the burn wound, invasion of viable tissue deep to the burn eschar, and thrombosis of blood vessels invaded by bacteria at the margin of the necrotic zone. Septic emboli with fibrous organization were seen in pulmonary artery branches in all lobes of the lungs. There were foci of necrosis throughout the lungs in which bacterial proliferation was visible within the walls of arteries, and these necrotic areas were surrounded by massive pulmonary hemorrhage and edema. A direct hematogenous route of spread of infection from the skin to the lungs seemed clear in this case. On the other hand, airway obstruction is known to predispose to pneumonia acquired through the airways, by preventing normal clearance of bacteria from the airways and by providing a favorable medium for bacterial growth. Multiple foci of airway obstruction are almost always seen at autopsy in burn patients.22 In another recent case, although pneumonia was not present, there was invasion of antibiotic-resistant Pseudomonas directly into the wall of a bronchus. The risk of infection is proportional to the extent of severe burns, the time elapsed before initiation of fluid therapy, the presence of metabolic alterations, the development of immunologic deficiency, the concurrence of trauma, the local evolution of wounds, and the age of the patient. An infection may begin in a burn wound, the respiratory system, the gastrointestinal tract, the urinary tract, the blood vessels, or from localized infection in any other area of the body. Although most serious infections in burn patients appear to be due to endogenous flora, and many derive from wound infections present at the time of admission, nosocomial infection is a constant hazard.23,24
The problem of burn wound sepsis is amenable to therapy. The strategy of excision of the potentially infected burn wound as early as possible, together with judicious administration of effective antibiotics, has greatly reduced the number of deaths due to infection. Coincidentally with the institution of early excision and grafting of burn patients in our institution, the incidence of burn wound sepsis as a cause of death declined dramatically. However, the problem of sepsis persists despite the best efforts in prevention and treatment. Certain organisms can evade the best current efforts at management, and have caused death in recent cases, including bacteria resistant to all available antibiotics.25 Those patients who are referred for therapy more than 1 week after burn injury often have extensive invasive wound infection and sepsis, which may be difficult or impossible to eradicate. Other microorganisms also can cause life-threatening infection in burn patients. Fungal infection is a continuing problem in patients with large burns, despite the development of more effective systemic antifungal agents.26 In one recent case of apparent systemic fungal infection, invasive and systemic dissemination of a pseudofungus of the genus Oomyces was demonstrated.27 Molecular methods (RT-PCR) were used to identify the organism in this case. It seems likely that the increased availability of molecular diagnostic testing of infectious agents may lead to recognition of additional previously unrecognized causes of invasive infection in burn patients. We have also experienced cases in which acquisition or reactivation of herpes virus infections led to major tissue injury. The risks of infection of a victim of a large burn are somewhat similar to those of an immunosuppressed transplant patient.
Coagulopathy
The burn wound has procoagulant effects and may induce coagulation throughout the circulation (DIC, disseminated intravascular coagulation).28,29 Tissue necrosis, particularly lethal injury to endothelial cells with exposure of subendothelial collagen, and release of tissue thromboplastin, can activate coagulation and lead to coagulopathy. Generation of thrombin within the circulation leads to generation of fibrin peptides and stimulates acute inflammatory reactions, including increased vascular permeability and upregulation of adhesion molecules on neutrophils and endothelial cells.30 Generation of fibrin degradation products may be sufficient to interfere with normal thrombosis, and thrombocytopenia can develop in response to abnormal intravascular fibrin generation.31 Activation of the kinin system can stimulate further abnormal vascular permeability and hypotension.20 Consumption of coagulation factors can lead to abnormal bleeding, which can cause extensive tissue injury secondarily. It is important to note that the acute-phase response to burn injury includes increased synthesis of fibrinogen and factor VIII. During the first 3–10 days after burn injury, patients often have greater than normal clotting activity. This may increase their susceptibility to the development of DIC, especially if sepsis supervenes. It also implies that laboratory testing of levels of fibrinogen and factor VIII may yield normal values even in the presence of abnormal consumption of these factors. When disseminated intravascular coagulation occurs, coagulation factors are depleted, including antithrombin.32 There is evidence that administration of recombinant antithrombin is beneficial when administered early in the course of treatment of severe burns.33 When disseminated intravascular coagulation occurs in the patient’s terminal course, microscopic fibrin thrombi are seen in many organs at the time of autopsy, most commonly in the lungs, the skin, the kidneys and the gastrointestinal tract.14,34
Integumentary system
The skin is the site of initial injury in burn patients, and many of the events that lead to dysfunction or failure of other organs begin in the skin. Thermal injury rapidly produces irreversible injury and cell death in epidermal keratinocytes, in the epidermal appendages, including hair follicles and their attached sebaceous glands and sweat glands, and in the connective tissue cells of the dermis. In many cases the burn wound excised within 48 hours of injury shows that the entire dermis and all of the hair follicles are necrotic, but that much of the subcutaneous adipose tissue remains viable. It appears that the greater insulating capacity of adipose tissue protects it to some extent. In some cases, of course, the necrosis of the initial thermal injury may extend deep into the subcutaneous tissue. In extreme cases, the underlying skeletal muscle may become necrotic as a result of thermal injury. Necrosis of skeletal muscle is especially prominent in the case of electrical injury, in which more heat may be generated adjacent to bone than near the body surface. An interesting observation is that there is often a band-like infiltrate of degenerating polymorphonuclear neutrophils in the midst of a totally necrotic dermis. This suggests that the boundary between necrotic and viable tissue may have extended deeper after the initial burn injury and its inflammatory response. There is experimental evidence that burn wounds often evolve from an initial level of necrosis to a deeper level, even from second to third degree, as a result of poor perfusion of the tissue immediately deep to the initial burn injury.35 This process of vascular stasis deep to the burn is undoubtedly due in part to the rapid loss of intravascular fluid from the damaged capillaries and venules just below the necrotic burn wound. In addition, there is evidence that neutrophils contribute to this process of burn wound extension, most likely by adhering to endothelium and to each other, with resulting obstruction of the microvasculature.36
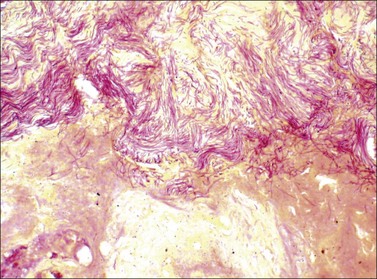
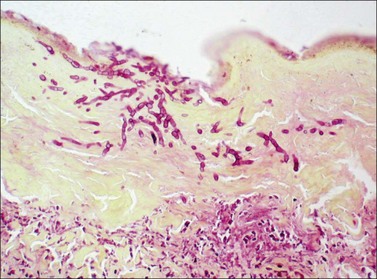
It is important to assess the presence and extent of infection within the burn wound, both by examination of wounds excised therapeutically and by biopsy of suspicious areas in open foci after grafting procedures. A high index of suspicion serves the burn patient well. All biopsy and excision specimens in our institution are sampled and studied histologically with stains for bacteria (Brown and Hopps) and fungi (methenamine silver). In large excision specimens, samples are taken from sites of especially deep tissue injury and sites that show abnormal discoloration of dermal or subcutaneous tissue. When infectious microorganisms are found, it is important to determine their location with respect to the boundary between living and necrotic tissue. This boundary may be irregular. It is generally distinct and marked by inflammation in wounds several days old, but may be somewhat indistinct in very fresh specimens, as karyolysis takes some time to develop in burn wounds. As noted above, wound infections generally begin with colonization of the skin surface and proliferation of organisms on the surface, often with extension into hair follicles, followed by growth within the necrotic tissue. Both the coagulum on the surface and the necrotic epidermis and dermis are considered part of the burn eschar. Growth within necrotic tissue is considered evidence of tissue infection, however, and potentially more dangerous than growth on the surface of necrotic skin, under a layer of fibrin and debris. Even when quantitative cultures show more than 105 bacteria per gram of tissue, when careful histologic study shows that the organisms are limited to the skin surface or the superficial necrotic tissue, the risk of sepsis appears to be low. Such growth on or in necrotic tissue, however, does set the stage for invasion of viable tissue. The finding of clusters of bacteria or fungi within viable tissue implies a serious risk of sepsis and further tissue invasion. Bacterial invasion of viable tissue is quite apparent by histologic study of appropriate tissue samples (Fig. 44.1). Invasive fungal infection presents a somewhat different pattern, in that there is often a wavefront of necrosis that accompanies fungal invasion (Fig. 44.2). Thus the presence of fungal hyphae extending to a boundary between necrotic and viable tissue is considered evidence of fungal invasion of viable tissue. On this basis, infections identified within burn wounds are reported as surface colonization, invasion of necrotic tissue, which may be superficial or deep, and invasion of viable tissue. The responsible surgeon is called immediately when invasion of viable tissue is found. If the level of clinical suspicion is especially high, frozen sections have been found useful for this determination, using a tape transfer device to facilitate handling of these difficult specimens, with confirmation of the results with routine sections on the following day. Diagnosis of viral infection of the skin is achieved most efficiently by preparation of smears from scrapings of freshly opened vesicular lesions or the bases of recently ruptured vesicles, and direct immunofluorescence testing.
A fortunately rare complication involving the skin of patients with large burns is the development of squamous cell carcinoma during the late phase of recovery. This so-called ‘Marjolin ulcer’ shows extensive local invasion but generally can be controlled with adequate local excision.37–39
Respiratory system
Respiratory failure, defined as inability to maintain adequate oxygen saturation while administering 100% oxygen by ventilator, has been found to be the immediate cause of death in some burn patients. The causes and mechanisms of respiratory failure are multiple and will be addressed separately, although in many cases more than one mechanism operates. Direct thermal injury to the trachea and bronchi probably does not occur, except in patients exposed to large amounts of steam. In addition to the problems listed below, patients may develop problems related to the airways such as pneumothorax or interstitial emphysema, aspiration of gastric contents, pulmonary embolism, and non-specific pulmonary edema due related to increased venous pressure. Lesions of the lungs, which may be multiple, are often found at autopsy in burn patients.40
Infection
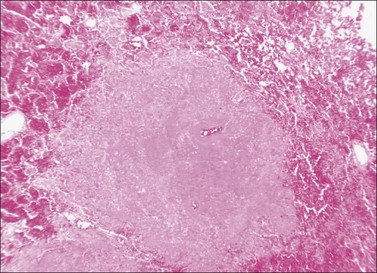
In patients who have clinical evidence of sepsis at the time of death, extensive infection of the lungs is commonly present and may represent a terminal event. Fatal pneumonia is most often seen as a consequence of infection with a highly resistant bacterial strain, an invasive fungus, or in a compromised host with renal failure or some other cause of severe immunodeficiency. Virulent and antibiotic-resistant strains of Pseudomonas may produce an angioinvasive infection in the lung, with massive proliferation of bacteria within the walls of pulmonary arteries and ischemic necrosis of segments of lung tissue (Figs 44.3–44.5).41 This pattern is similar to that of ecthyma gangrenosum of the skin.42,43 A similar angioinvasive pattern of pulmonary infection can be seen with generalized infection due to Aspergillus or similar filamentous fungi.