METHODS TO ASSESS SKIN BARRIER INTEGRITY:
EFFECTS OF CLEANSING PRODUCTS
Guojin Lu, Roger L. McMullen, and David J. Moore
Ashland Specialty Ingredients
ABSTRACT
Protecting skin barrier integrity is vital to maintaining the health of human skin. While providing skin hygiene, surfactant-containing cleansing formulations can damage the skin and reduce its natural defensive barrier function. Such formulations can also cause dry, flaky, or itchy skin as a result of irritation due to surfactant absorption and penetration. Several mechanisms have been proposed to describe the interaction of surfactants with the stratum corneum (SC). These include protein denaturation, natural moisturizing factor (NMF) removal, selective SC lipid removal, and disruption of SC lipid organization leading to increased permeability through the SC.
In this chapter, we discuss the effects of cleansing stresses (e.g., surfactants, pH, and cleansing product temperature) on skin barrier integrity. We also describe some practical methods that can be used for evaluating the extent of skin barrier damage induced by cleansing formulations, as well as the advantages and disadvantages of each measurement technique.
a. Skin structure and functions
b. Cleansing and commonly used surfactant systems in cleansing formulations
c. Skin damages by cleansing products
11.1.2 Physicochemical interactions between surfactants and skin
a. SC protein binding, denaturation, dissolution,
and SC swelling
b. SC lipid extraction and selective removal
c. Disruption of SC lipid organization/structure
and change of lipid composition
11.1.3 Approaches and methods to assess the effects of
cleansing stresses on skin barrier integrity
a. Surfactant-skin/SC interactions
1. Sensory testing and substantiating instrumental methods
2. Microscope, video microscopy for skin surface topography
3. Skin penetration/permeability by Franz cell and
impedance measurements
4. Mechanical behavior of skin
5. Bioengineering methods to measure water flux
and water content of skin
b. Surfactant-lipid interactions
1. Differential scanning calorimetry (DSC)
2. Vibrational spectroscopy (FT-IR)
3. Quartz crystal microbalance (QCM)
a. Skin structure and functions
In addition to being the largest organ of the human body, the skin is the interface between the organism and the external environment and protects the body from external insults. In the design of skin care products or formulations, the ultimate consumer experience, as well as skin health and appearance, are determined by formulation ingredients and their material properties. The physical and chemical activity of these ingredients and their interactions with each other influence both skin/stratum corneum (SC) structure and their biochemistry. In order for the cosmetic scientist to improve skin condition or reduce the skin damage caused by cleansing products, an understanding of skin structure and function is essential. This is especially true concerning the basic physiology of the outer epidermal layer, SC.
There have been many articles that reviewed skin structure and functions in detail, which is however not the primary focus of this chapter1–3. Briefly speaking, skin is a complex multilayered organ consisting of three components. Looking from the outside towards the inside, these components are the viable epidermis, the dermis, and hypodermis. Major skin functions include: i. the protective and defensive barrier (its vital function) to prevent uncontrolled water loss and to protect the body from a variety of environmental insults (e.g., abrasion, microorganism invasion, UV or other radiation damage, exogenous chemical agents such as surfactants and solvents, and other daily insults); ii. thermo-regulation function through blood flow and sweating to maintain normal body temperature; iii. immune system function; iv. sensory function via the nervous system; and v. psychological function for skin visual appearance and social acceptance.
In practice, it is generally accepted that topical skin care is primarily concerned with protecting the skin barrier4. A widely employed analogy for the SC organization is a brick-mortar (corneocyte-lipid) model5. Overwhelming evidence indicates that SC lipid composition and organization governs skin permeability and plays a critical physiological role in regulating water loss/uptake through the skin. In healthy skin, lipids are typically in a lamellar bi-layer crystalline state. The organization of the SC lipid membranes is very complex and is comprised of three major components: free fatty acids (FFA), ceramides (CER), and cholesterol (CHO). Together they form the highly ordered lipid phase of the SC.
The SC lipid matrix, and also the mixed SC lipid model systems that have been widely used to study the SC, do not exist as homogenous or uniformly mixed single phases, but rather form separate domains (and bi-layer phases) in the SC lipid membranes6–8. Although the exact ratio of the phases has not been determined experimentally, fluid (liquid crystalline) and solid phases (both hexagonal and orthorhombic) of lipids do coexist, which is a crucial requirement for the barrier properties and flexibility of the SC lipid layers.
b. Cleansing and commonly used surfactant systems in cleansing formulations
As part of their daily routine, consumers frequently use surfactant-containing cleansing products such as soap bars, detergents, as well as liquid hand soaps, body washes, cleansing lotions, liquid face washes, (hand) dish wash, etc. These surfactant-based preparations are efficacious cleansing systems that help remove unwanted materials such as dirt, oil, sweat, debris, pathogenic microorganisms, and other excess body and cutaneous serums from different substrates (skin, hair, fabrics, dishes, etc.). Cleansing efficacy is very important for hygiene and is achieved by means of surfactant properties such as solubilization/emulsification of grease in liquids, and dispersion or suspension of solids in liquids. These properties arise from the ability of surfactants to concentrate at the interface, leading to a reduction in interfacial tension. Because of their characteristics, surfactants are among the most important cosmetic ingredients.
Surfactants are amphiphilic (two-part) molecules that are composed of a hydrophobic portion (tail group, almost always a hydrocarbon structure) and a hydrophilic portion (head group). When the solution concentration of a surfactant reaches its critical micelle concentration (CMC), surfactant molecules tend to form association structures of dynamic aggregates such as micelles. These micelles are typically spherical in nature with the hydrophobic portion facing inwards. As a result of this structure, they can dissolve oils easily by incorporating oil within the inner portion of the micelles and by associating their hydrocarbon tail with the oil surface, thereby causing the solubilized oil to lift up from the surface of the substrate. Depending on the composition or charge of the surfactant molecule (head group), surfactants can be classified as anionic, cationic, nonionic, and amphoteric or zwitterionic (the charge of the head groups changes with pH) surfactants.
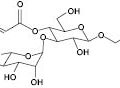
For most skin-cleansing formulations, anionic surfactants with hydrocarbon chains of various length especially those with C12 chain length are typically used as primary surfactants (e.g., sodium lauryl sulfate [SLS] or sodium laureth sulfate [SLES]) due to their desirable detergency, foaming, and lather features. Amphoteric surfactants, such as betaines, are normally used as co-surfactants in liquid cleansers that are milder with respect to the skin barrier. Nonionic and cationic surfactants are less frequently used in skin-cleansing systems. With regard to the extent of harshness, the following order is well known: anionic surfactants (highest) > amphoteric surfactants > nonionic surfactants. Among the anionic surfactants, besides soap surfactants that are originated from animal or plant sources (e.g., SDS [or SLS], ALS, or SLES), synthetic detergents (syndets) are also frequently used as they are milder surfactants. For a surfactant with given chain length, the larger the head group size, the lower its tendency to cause skin damage due to steric hindrance. Other factors that affect harshness of a surfactant include its critical micelle concentration (CMC) and micelle size.
c. Skin damages by cleansing products
Due to personal cleansing activities, human skin is constantly exposed to surfactants, which are well known to compromise the essential barrier function of healthy skin. Skin barrier damage can be accompanied by after-wash skin tightness, dryness, scaly skin, or even erythema, irritation, and itch due to surfactant absorption and penetration into the skin. These surfactant-induced, undesirable visible manifestations of skin and uncomfortable skin feeling are greater when the ambient temperature and humidity levels are relatively low in the winter environment. These undesirable side effects are also interrelated.
In addition to adverse effects, the alteration of skin barrier function by surfactants can be managed to enhance the penetration of topically applied materials, such as transdermally delivered drugs. In the case of surfactant use, a careful balance has to be maintained between maximum drug penetration enhancement and minimum skin irritant response, since any surfactant that has the capability to reduce the cutaneous barrier function for certain drugs could also enhance the permeation of other ingredients, including any excipients used as well as the surfactants themselves.
To understand the interactions of surfactants with the skin and their effect on skin barrier integrity—especially with the outer SC—it is essential to develop qualitative and quantitative measurement methods (both in vitro and in vivo) to predict, evaluate, and demonstrate the effect of different surfactant chemistries, formulation ingredients, and cleansing conditions.
In this chapter, we discuss surfactant-skin interaction mechanisms and the effects of cleansing stresses (e.g., surfactants, pH, and cleansing product temperature) on skin-barrier integrity. We also describe some practical methods for evaluating the extent of skin-barrier damage induced by cleansing formulations as well as the advantages and disadvantages of each measurement technique. We conclude with a discussion of employing these methods to assess the benefits provided by harshness-reducing technologies applicable in skin-cleansing formulations.
11.1.2 PHYSICOCHEMICAL INTERACTIONS BETWEEN
SURFACTANTS AND SKIN
As a system having a heterogeneous surface as well as a bulk material phase, the skin is very complex and surfactants applied to its surface can interact with many different targets in the skin structure in multiple ways. Only a few skin components will be discussed in this chapter: the SC (including intercellular SC lipids and dead cells of protein-containing corneocytes), keratin, and living cells of the epidermis.
It is generally accepted that surfactant-SC interactions and disruption of skin-barrier properties occur when surfactants have penetrated into the skin barrier. Several mechanisms have been proposed regarding the subsequent interaction of surfactants with the SC and skin components. These include, but are not limited to: enzyme disruption, skin permeation and penetration, protein denaturation and dissolution, SC swelling that causes enhanced water penetration, and removal of natural moisturizing factor (NMF) from corneocytes9–10. Such insults lead to reduced water-holding capacity and dry skin, selective SC lipid extraction or removal, and disruption of SC lipid organization/structure. They can also lead to a change of lipid composition, which generates an increased permeability through the SC lipid bi-layers that are responsible for the SC barrier function11–12. The above phenomena may also trigger some biological responses from the skin, such as the scenario described in the “dry skin cycle” model by Rawlings13. If the cleansing stresses (e.g., surfactant concentration, application period, pH, temperature, etc.) are strong enough, or the skin is not rinsed sufficiently, surfactant residue left on the skin will continue to penetrate into the viable epidermis, and perhaps even into the dermis. This process will cause further disruption of living skin cell homeostasis, ultimately leading to an inflammatory response. It is worth noting that the above-mentioned mechanisms are not independent from each other but rather related in a cycle.
Most of the mechanisms described above also involve water loss, either directly or indirectly. In other words, surfactant-based cleansing products cause water loss and reduce the water-holding capability of the SC by disturbing the skin’s normal mechanisms for regulating water content14. In fact, maintaining of optimal water content is a key factor (if not the most important one) for sustaining the practical function of the SC15–16.
a. SC protein binding, denaturation, dissolution, and SC swelling
Surfactant interaction with skin proteins can adversely affect skin hydration and viscoelasicity. It is widely reported that surfactants can bind to skin keratin with high affinity17–22. This binding reduces the ability of skin protein to bind and retain water, thereby causing dry and tight skin. This binding could also lead to the development of repulsive forces, causing some protein denaturation (separation of the protein matrix, uncoiling of the filaments, and exposing more water-binding sites), SC conformation change, and transient swelling/hyperhydration. These events are often followed by water evaporation causing skin drying17–18. This effect is thought to saturate at about the CMC since protein binding and denaturation are associated with surfactant monomers, although this hypothesis remains controversial11, 19–22.
In general, surfactants that bind more strongly to SC proteins have a higher potential to cause significant protein denaturation and severe skin-barrier damage. For anionic surfactants, the skin swells as water content increases with increase in charge—protein unfolding and dissolution may occur. An increase in water uptake only hydrates the skin temporarily, while eventually it will reduce the skin’s water-holding capability due to surfactant adsorption onto protein hydration sites. After rinsing, as the water evaporates, the hydration level of the skin is lower than the preexposure level. As the result, the skin will be even drier than before cleansing due to additional water evaporation and SC swelling. For other surfactant systems, there is little change with increase/decrease in surfactant charge and therefore minimal SC swelling is observed. Indeed, SC swelling can be reduced by the addition of secondary surfactants (such as betaine) even though the total surfactant concentration is increased by mixing23. This phenomenon can be explained by the reduced binding of the harsher surfactant due to the competition between them for binding sites, and the lowering of the CMC by the addition of a surfactant with lower CMC. Another way to reduce SC swelling is to increase the size of the head or polar group of surfactant, which will provide an additional barrier in the form of steric hindrance.
Surfactant and even water adsorption on the SC may dissolve some components of the water-soluble natural moisturizing factor, leading to a reduction in their levels in skin24. NMF is a heterogeneous mixture of hygroscopic amino acids in the corneocytes and it is one of the major components responsible for water holding (other two major water-holding SC factors are intercellular lamellar lipid organization and corneodesmosome function)25. NMF adsorbs water from the atmosphere to provide sufficient hydration to help keep skin flexible and smooth, as well as to support a variety of enzymatic reactions in the dermis. It is very water soluble and can be very easily leached by water if the protecting lipid bi-layers surrounding the corneocytes get damaged, as for example, by surfactant exposure. Consequences of reduced NMF include reduced hydration of the SC, reduced activities of SC enzymes leading to reduced desmosome breakdown, increased stiffness of the SC leading to the possibility of cracking around joints, and erythema due to inflammation associated with an undesirable biochemical response.
b. SC lipid extraction and selective removal
SC lipids are the major barrier to permeability of the SC and play a critical role in skin functions ensuring cellular cohesion, skin-barrier properties, water-holding capability, and normal healthy-looking skin. Indeed, skin or SC with disordered lipid structure or abnormal composition would be more water permeable and more vulnerable to external attacks. Mainly due to the effect on the lipid layers, surfactant exposure would increase the ability of exogenous compounds to penetrate the skin and to increase water loss through the skin.
It is reported that lipid extraction is possible above the CMC, and cholesterol and free fatty acids (not ceramides) are generally removed24, 26–29. In one study, Loden concluded that lipid extraction/solubilization was caused by surfactant micelles and therefore is concentration dependent30. When the SDS concentration is relatively high, more surfactant molecules are available for skin binding and penetration and thereby “consume” the lipid matrix. This concept is used to explain the mechanism of damage to SC by surfactants at concentrations above their CMC21, 31.
The solubilization and removal of SC lipid by a large amount of absorbed surfactant molecules has been reported in lipid films32. Lipid content in the skin SC is normally observed to decrease because of delipidization after cleansing with soap products33. In a study of recovery in SDS-treated skin by daily topical application of SC lipid fractions, Imokawa and colleagues strongly supported deficiency of intercellular lipids as an essential mechanism in surfactant-induced skin damage27. The concept of disruption of the lipid matrix (degreasing) is the dominant hypothesis for explaining the damage induced in SC lipid organization by surfactants. A few groups have challenged this idea of lipid extraction by conducting experiments suggesting that only very small amounts of lipids are removed from the SC by surfactants, even at high concentrations (above CMC), if the surfactant treatment time is not excessive (less than a few minutes)28–29.
c. Disruption of SC lipid organization/structure and change of lipid composition
While extraction of SC lipids by surfactants remains controversial and may not be the critical factor affecting lipid barrier function, alterations in lipid phase behavior and organizational structure, and disruption of the integrity of the lipid bi-layer barrier have attracted more attention. Surfactant exposure can cause expansion of intercellular spaces of the lipid lamellar structure and surfactant molecules will intercalate into the lipid network34. It is also reported that anionic surfactants induce intercellular lipid structure disruption, disordering, and, possibly lipid fluidization32, 35. Changes in lipid organization will impact skin irritation and alter the penetration of irritant molecules in the SC. This process also includes affecting desmosomal cleavage between corneocytes9.
Prolonged water and surfactant contact, although very different in their chemical nature, are both deleterious to the skin barrier since they disrupt SC lipid structure24, 36. In many situations human skin is exposed to both stresses, as for example during cleansing, as well as in many workplace environments. Lipid disruption not only leads to increased water loss through the skin, but also enhances penetration of other chemical substances including toxic and irritating compounds through the skin.
Long-term exposure to surfactants will also modify the lipid composition and cause phase transitions and changes in the overall phase behavior of the lipid bi-layer systems37. Lipid composition is of paramount importance for the SC organization and its barrier properties38–40. In fact, it is known that lipid composition in the SC is different between individuals, between anatomical skin sites, and a function of skin damage and diseases41. The alteration of the ratio of the three major lipid components may also cause phase separation of lipids at the surface of the stratum corneum and result in crystallization of the lipid matrix42. These phase changes in turn will affect the water-holding capability and barrier properties of the SC, causing dry skin and/or skin irritation37, 43–44. Evidence indicates that diminishing amount of ceramide and increasing levels of fatty acid (nearly doubled) are related to soap-induced winter xerosis42.
Based on literature reports, SC lipid-surfactant interactions likely progress as follows: (1) initial water loss due to osmosis or surfactant-induced water swelling or hydration to open up the lipid structure; (2) surfactant penetration/insertion into the lipids that further alters lipid phase, structure, and even composition, leading to extra water uptake by the lipid layers; (3) after extended exposure to surfactant solutions for a long period, part of the lipid matrix is removed by dissolution and the lipid barrier function is further damaged, causing water loss and dry skin due to reduced water-holding capability; and (4) SC disruption by surfactants stimulates SC formation and this new formation typically proceeds too quickly, leading to abnormal architecture and inadequate barrier function thereby causing low hydration and abnormal desquamation (scaly appearance).
Another factor that can aggravate the deleterious effects of surfatcant-skin interactions is the pH of the cleansing formulation. It is well known that pH plays a very significant role in the alteration of skin intergrity by surfactants. The effect of pH on SC barrier function and skin permeability to drug molecules has been the subject of many studies9, 45–51. The results of these studies demonstrate that the enviromental pH to which skin is exposed has a significant impact on SC lipid composition and organization. Further it impacts SC cohesion, protein swelling, and skin irritation. Indeed, a predictable relationship between the solution pH and skin irratancy has been demonstrated, although it has been reported that the influence of pH on the chemistry of surfactant-based washing compositions is more important than that of the direct contribution of pH on the SC52.
Since the pH of a healthy SC is acidic (generally around pH 5), the attaction and penetration of basic surfactant solutions is more favorable. Under basic pH conditions anionic surfactants bind primarily by hydrophobic functional groups to hydrophobic sites on the skin to minimize the repulsion of negative charges, thus leading to swelling of the SC in the presence of more alkaline surfactants35. The effect of surfactant pH on SC was further demonstrated to negatively influence the barrier repair mechanism by the daily use of soap-based cleansers with greater pH53.
Soap-based cleansers are alkaline in nature (pH 10, e.g., for regular soap bars) while milder synthetic detergents (syndets, such as Dove bar) are mostly neutral (pH ~7 for Dove soap bar). The fact that detergents with higher pH are less mild is not surprising since there is more binding to keratin by anionic surfactants52. It has been reported that alkaline pH alone, in the absence of surfactants, induces an increase of SC swelling and irreversible lipid phase changes in SC9, 45, 54.
11.1.3 APPROACHES AND METHODS TO ASSESS THE EFFECTS
OF CLEANSING STRESSES ON SKIN-BARRIER INTEGRITY
Requirements from consumers have stimulated the development of milder cleansing products, which makes it difficult to differentiate minor variations between products. Exaggerated and more progressive application tests (i.e., “accelerated” testing), where test conditions are modified to be harsher (e.g., more concentrated, elevated temperature, longer exposure period, etc.), are usually conducted to enhance the skin reactions.
For clinical tests utilizing human subjects, the procedures are regulated by ethical recommendations and test guidelines to ensure safety and standardization. More details may be found in a published series of testing procedures and guidelines by the European Group for Efficacy Measurements on Cosmetics and Other Topical Products (EEMCO) for tests related to different skin properties, such as dry skin and xerosis55–56, skin hydration by electrical methods57, TEWL in cosmetic sciences58, skin surface pH59, tensile functional properties60, skin color61, skin microcirculation62, and skin topography63.
While it is the most straightforward method to assess the harshness of skin-cleansing products, consumer perception and expert grading is not objective and, therefore, not always reproducible or reliable35, 64. As a result, a large number of subjects are required and different consumer habits and practices have to be considered. It is also difficult to distinguish the small differences between relatively mild cleansing products using such methods. These problems make it difficult, expensive, and time consuming to directly compare skin-cleansing products solely by consumer perception tests. Consequently, various exaggerated methods and approaches have been developed for formulation screening and product development, leaving consumer testing as a tool mostly used for claim substantiation of final products.
Noninvasive biophysical and bioengineering methods offer insights into the different aspects of skin structure and functions. These methods are primarily based on sophisticated scientific instrumentations and have been used to measure skin characteristics following environmental exposure (e.g., UV exposure) and cosmetic treatment (e.g., surfactants, skin care products, etc.) as well as monitoring skin aging and diseases. They are sensitive, easy to use, fast, mostly portable, and not costly. They are practical approaches that can be used to monitor and assess topical treatments for in vivo human skin in real time and to obtain objective, quantitative, and reproducible information on skin conditions after surfactant challenge.
In the last several decades, various experimental approaches have been utilized to better serve different application needs. There are in vivo methods where human skin is subjected to various imaging and instrumental methods. Often tape stripping is applied to provide more in-depth information. Frequently in vitro approaches are employed. In these cases, full thickness human or porcine skin, isolated stratum corneum, or extracted lipids may be used. Porcine skin is commonly employed due to its physiological similarity to human skin. Other than real skin samples, some model materials have been recently developed to study surfactant interactions with skin, SC, lipid, or proteins. These include lipid models, protein models, synthetic membrane substrates (vitro skin, vitro corneum, episkin®, silicone membrane, etc.).
To model the interactions of surfactants with the skin and their effect on skin-barrier integrity, especially with a focus on the outer SC, it is essential to develop qualitative and quantitative measurement methods (both in vitro and in vivo approaches) to predict, evaluate, and demonstrate the effect of different surfactant chemistries, formulation ingredients, and cleansing conditions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree