(Author: Charles Warren)
6.2 Skin Lightening, Whitening, and Brightening:
An Overview of Approaches, Key Ingredients, and
Formulations for Enhancing Skin Appearance and
Correcting/Minimizing Common Skin Pigmentation
Disorders (Authors: Eva Patel and Gogi Sangha)
6.2.2 Common Skin Pigmentation Disorders
6.2.3 Triggers for Hyperpigmentation
6.2.4 Pathway to Hyperpigmentation
6.2.5 Formulating Ingredients—A Plethora
of raw materials and how they come into play
6.2.6 Formulations for Individual Skin Conditions
6.2.7 Claim/Regulations in USA
6.3 Sunscreens (Author Charles Warren)
6.4 Antiperspirants / Deodorants (Author Charles Warren)
6.5 Acne, Oily, ad Aging Skin Product Formulation
(Author Mark Lees)
a. The Acne-Prone and Clog-Prone Skin: A Client Profile
6.5.2. Review of factors in acne development
b. The Development of Acne Lesions
d. Topical and Environmental Factors
6.5.3 Management of acne-prone skin
c. Avoidance of Acnegenic and Comedogenic Products
6.6 Face and Body – Masks / Scrubs (Author Charles Warren)
6.7 Shaving Preparations: Pre and Post (Author Charles Warren)
Authors: Germain Puccetti, Nevine Issa, and Hani Fares
6.8.1 Color Cosmetics and the Consumer
Perspective
6.8.3 Lipsticks and Lip-Glosses
6.8.5 Skincare Actives in Foundations and
Lipsticks
6.9 Hair Care:
Formulating Wisdom Category by Category
Ingredients, Formulation and Efficacy Evaluation
(Author Carrie Shipley, Applications Scientist,
Grain Processing Corporation)
Section I: Typical Shampoo Ingredients
6.10.2 Rheology and Viscosity Modifiers
6.10.3 Other Shampoo Ingredients
Section II: Hair-Cleansing Mechanism
6.10.7 Cleaning of Solid Particulates
6.10.9 Efficacy of Soil Removal by Shampoos
6.10.11 Cleaning of Quaternary Ammonium Compounds
6.10.12 Cleaning of Polymeric Residue
6.10.13 Effect of Shampoos on Hair
Section III: Shampoo Evaluation
Section IV: Future Trends in Shampoos
6.11 Hair Styling (Author Charles Warren)
a. Nonpressurized Styling Products
b. Pressurized Styling Products
6.12 Specialty Styling Products (Author Charles Warren)
6.13 Permanent Waving (Author Charles Warren)
6.14 Conditioners/Treatments (Author Charles Warren)
6.15 Hair Colorants and Protection (Author Padmaja Prem)
6.15.2 Fundamentals of Hair Coloring
6.15.3 Factors Influencing Color Fading and Color Removal
6.15.5 Color Vibrancy and Shine
6.15.6 Remedies for Color Protection, Vibrancy, and Shine
6.15.7 Ingredients and Products for Color-Treated Hair
6.16 Reactive Hair Care Products (Author Charles Warren)
6.17.1 Functionality/Performance
6.17.2 Marketing Requirements/Expectations
6.17.3 Manufacturing Requirements/Expectations
6.17.4 New Raw Materials, Bases, Forms
Exactly what is a “formulator”?
First and foremost, a formulator is a good scientist. A formulator has to have a good technical sense and a fundamental understanding of chemistry, biology, physics, statistics, and consumer sciences. The deeper and more complete the technical understanding, the better and more creative the formulating can be.
A formulator needs to understand the chemical relationships among the raw materials being used in whatever category the formulating is being done. Understanding the chemical function of the raw materials and their interactions with other raw materials allows the formulator to choose the best combination(s) for the particular end formula.
Likewise, a formulator has to understand the physics and physical properties of the desired end product – dictated by the category, the manufacturing requirements, the filling and dispensing properties and, most importantly, the physical characteristics desired by the ultimate consumer.
The formulator needs to understand the medium upon which the formulation is to be used—hair or skin and how that medium is impacted (positively and negatively) by the application of the particular formula. In addition, the formulator must understand the potential microbiological perils that can be introduced into the formula and the safest, most effective ways of preventing those contaminations from affecting the formula.
The formulator needs to understand how the formula is put together during manufacture and filling and how those operations impact the properties of the formulation. Additionally, the formulator needs to understand the relationship between the formula and the primary packaging, with special attention to how the formula will be dispensed from the package. Many formulators have had the unpleasant surprise of watching their formula become too thick as it is forced through a small orifice or, worst of all, cannot be dispensed from the package selected.
Consumer science is important for the formulator because the consumer is the ultimate judge of a product and, ultimately, its success in the marketplace. Understanding consumer testing and the statistical analysis of consumer data will always be of great benefit to the formulator. Dealing with consumer expectations is always challenging, but those expectations are extremely important to formulation and the ultimate marketing of a successful product.
Secondly, a good formulator needs to be an artist. Translating the “wish-list” from Marketing or the Consumer into a tangible consumer product is truly an art. Understanding the aesthetic requirements and the particular combinations of raw materials that deliver against those requirements is definitely an artistic endeavor and one that a good formulator must really take the time to pursue.
So, a formulator is a combination of scientist and artist, a good observer of both technical and aesthetic properties, a good listener to all interested partners—Marketing, the Consumer, Manufacturing, etc., and an objective and unbiased evaluator of information.
A formulator needs to understand the category in which the formula is being developed
In skin care, the categories are usually split between hand/body and facial products. In hand/body care there are cleansers, moisturizers, and a group of
miscellaneous products, such as sunscreens. In facial care there are cleansers/scrubs, moisturizers, and treatments, such as acne treatments, fine-line and wrinkle treatments, masques, etc. There are other groups of products also, such as perfumes/fragrances, pigmented cosmetics, nail products, and products related to shaving and depilatory products.
In hair care, the categories are: shampoo, conditioner/treatments, styling and reactive products. The formulator also needs to understand the targeted consumer within the category. A general market shampoo for European hair would have different technical and aesthetic requirements than a shampoo for African/Caribbean hair or a shampoo for Asian hair.
An understanding of the existing environment, individual company, and competitive arena for each category is a good starting point. If the formulator is working for a company, a thorough understanding of the existing formulations within the category is important in understanding the palette of raw materials and familiarity with manufacturing. A review of the competitors in the category will provide insight into what is having success in the category and where there may be formulating opportunities.
This section will be devoted to providing assistance to the new formulator and review and refreshment for the experienced formulator.
FORMULATING WISDOM – CATEGORY BY CATEGORY
Author
Charles Warren
The skin is the largest organ of the human body. Below the outermost layer (stratum corneum) the skin is a living, biologically active organ. Unlike hair, healthy skin can repair itself and every 28 days actually replaces itself. It is the barrier between the internal body (75–80% water) and the external environment (significantly lower water content) and all of the perils it faces from that same external environment.
When formulating for skin care, there are two major categories: Hand and Body Care and Facial Care.
Hand/Body
Cleansers
The general categories of body and hand cleansers are soaps, bodywashes, and liquid hand washes. The function of all of these is to cleanse the body and hands of excess oils, particulates, and other substances adhering to or residing on the skin. Soaps are a special category, while bodywashes and liquid hand cleansers are very similar in form and composition.
The classic definition of a soap is an ionic salt of a long-chain fatty acid. A long-chain fatty acid, such as stearic acid, is neutralized with an alkali material to a stearate. When neutralized with sodium hydroxide, sodium stearate results; with ammonium hydroxide, ammonium stearate, etc. These stearates will emulsify the oils on the skin and adhere to particulates and then allow them to be rinsed away with water. Formulating and manufacturing soaps is a specialized endeavor and is usually handled by suppliers or manufacturers highly skilled in this medium.
Bodywashes and liquid hand cleansers are more prevalent and more reasonably formulated and manufactured by personal care formulators/manufacturers. In general, these are aqueous solutions of primary surfactants (e.g., laureth sulfate), secondary surfactants (e.g., betaines), foam modifiers (e.g., cocamide MEA), conditioning agents (e.g., hydroxypropyl trimonium chloride), aesthetic modifiers such as sodium chloride (viscosity), color and fragrance, and microbiological preservatives. If the product is to be opaque or pearlescent, materials such as glycol stearate can also be used. Once an acceptable base formulation of surfactants and modifiers is identified, a wide variety of variables can be developed by changing colors, fragrances, and other additives to support positions and claims. These products can be easily formulated to dispense from tubes, bottles, through pumps, etc.
Moisturizers
The largest groups in this category are the hand and body lotions. These formulations are typically oil-in-water emulsions. Their primary function is to develop a light, oily layer on the surface of the skin to prevent water from leaving the lower layers of the skin, causing the surface to dry, flake, and develop a dull appearance. In addition, the other ingredients will help bind and retain moisture in the surface layers of the skin, leave the skin feeling soft and pliable, and impart a pleasing look and feel to the skin for an extended period of time.
A general overview of the ingredients used in these products would include:
- • Water – the mobile phase of the emulsion
- • Glycerin – a humectant that will reside in the stratum corneum and bind and hold water to itself
- • Isopropyl palmitate – a light oily material to lubricate the skin and help occlude for water retention
- • Petrolatum – the best occlusive agent to prevent trans-epidermal water loss (TEWL) and provide body and stiffness to the lotion
- • Silicones/cyclosilicones – help improve spreadability and feel of the lotion on the skin
- • Distearyldimonium chloride – a skin conditioner to help make the skin feel soft and pliable
- • Cetyl alcohol/cetearyl alcohol/ceteth-20 – common emulsifiers to hold the oil droplets in the emulsion
In addition, various other fatty esters, cationic polymers, and thickening agents can be used to improve the texture and feel of the lotion.
A word of caution – Certain claims related to protection and “healing” can quickly move a formula from a cosmetic product into the realm of over-the-counter drugs (OTC). OTC drugs have very specific requirements related to the levels of the active ingredient (e.g., dimethicone), the claims and exact wording of the allowable claims, stability and packaging testing, labeling, and manufacturing. A prudent formulator will review formulas and claims with Regulatory and/or Legal groups to ensure compliance.
Authors
Eva Patel & Gurpreet (Gogi) Sangha
ABSTRACT:
Skin-lightening products form a major segment in the cosmeceuticals market worldwide; and they carry with them the promise of flawless-appearing skin. This demand for “skin-fairness” products stems from the desire to eliminate hyperpigmentation, melasma, and post-inflammatory hyperpigmentation as well as a consumer interest to lighten overall skin tone through the use of skin lighteners, whiteners, and brighteners.
The continually growing demand for skin tone corrective treatments by the global beauty market has prompted scientists to develop an array of natural and synthetic raw materials that work on various pathways of skin pigmentation. Most skin lighteners currently in use are of botanical or natural origin and have multifunctional topical benefits. Such materials can also act as free radical scavengers for skin that has been over exposed to UVA and UVB solar radiation. These specialty ingredients can be formulated into topical solutions such as creams, serums, and lotions.
In the U.S., topical compositions containing hydroquinone are the only ones that regulations allow to make a skin-whitening and -lightening claim. Such compositions have been classified by the U.S. FDA as a drug. Many botanical, natural, and synthetic ingredients are available that may produce results similar to those of hydroquinone; however, current regulations covering compositions containing such ingredients are only allowed to make the claim of “skin brightening.”
TABLE OF CONTENTS
6.2.1 Definitions
6.2.2 Common Skin Pigmentation Disorders
6.2.3 Triggers for Hyperpigmentation
6.2.4 Pathway to Hyperpigmentation
6.2.5 Formulating Ingredients—A Plethora of raw materials
and how they come into play
6.2.6 Formulations for Individual Skin Conditions
6.2.7 Claims / Regulations for USA
Skin Lightening, Whitening, Brightening
Skin lightening has become a significant trend attracting the interest of technologists, product developers, and marketing/business leaders. Today, women, and even men, want to alter the tone and appearance of their skin to meet current regional standards of beauty or to address an underlying medical or cosmetic condition. Amazingly, skin whitening has become increasingly popular.
In the USA many people desire flawless and even-toned complexions; however, this is not the case in Asia. In this geographical area, it is a cultural issue that one has fair skin as it is a sign of beauty, wealth, and status. An article in The Economist titled “The Line of Beauty” (August 27th, 2011) suggests that good-looking people are more likely to succeed, be accepted by their peers, and get higher-paying jobs. In many countries, socioeconomic status depends on your skin color, as for example in India and Mexico. In India even your marriage is based on skin color, and the rising skin-tone consciousness continues to grow rapidly. From an epidemiological point of view, we find this trend-to-whiteness fascinating in view of the well-recognized global shift in the population towards darker-skin peoples.
Skin Lightening & Whitening/Skin Bleaching: A common drug claim for OTC/RX products containing Hydroquinone, as this is the only skin-lightening active ingredient that is recognized by the U.S. FDA.
Skin Brightening: Brightening is a term used throughout the skin care industry to classify formulations that brighten uneven skin tone and contain alternatives to hydroquinone. These alternatives can include botanicals, natural ingredients, peptides, and vitamins. Natural skin lighteners are also considered multifunctional ingredients that provide multiple benefits for the skin.
Hyperpigmentation/Melasma: Overproduction of melanin in the skin that is denoted by dark spots or patches
Hypopigmentation/Vitiligo: Areas of the skin that have lost their pigment completely when there are no longer functioning melanocytes in that area to create pigment, leaving white spots or patches on the skin
Melanogenesis: The process of melanin being formed in the melanocyte located at the dermal/epidermal junction in the skin
Melanin: The substance that gives our skin its color/pigment. This natural pigment is our skin’s protective shield against UV aggression. Dark skin tones contain more melanin in their skin and lighter skin tones have less melanin.
6.2.2 COMMON SKIN PIGMENTATION DISORDERS
- 1. Melasma/Chloasma – A condition that is typically induced from hormones internally. Also known as a pregnancy mask because most cases are seen in women postpartum. This condition can also be induced from menopause and even certain medications. MSH (melanocyte-stimulating hormone) is a natural hormone in our bodies and is linked to pigmentation and hyperpigmentation development in individuals with Melasma/Chloasma.
- 2. Post-Inflammatory Hyperpigmentation – This condition can be seen in the skin after inflammation has been present. Most commonly found in acneic skin conditions, this term is a broad category and includes “acne prone, reactive, oily skin type,” that experiences persistent breakouts and also post–aggressive skin care treatments or medical procedures.
- 3. Sun Damage – Sun is the number one trigger for hyperpigmentation, causing sun spots from years of excessive exposure to the ultraviolet rays. These are also known as photo damage or age spots.
6.2.3 TRIGGERS FOR HYPERPIGMENTATION
- 1. Sun (UVA/UVB rays, environmental damages, free radicals)
- 2. Hormones (internal unbalance, pregnancy, menopause)
- 3. Inflammation (blemishes, acne, injury, laser treatment, heat, medical procedures, chemical procedures)
- 4. Oral Medications (diuretics, birth control pills, antibiotics, anti-depressants, etc.)
- 5. Alcohol (aftershave, colognes, perfumes)
6.2.4 PATHWAY TO HYPERPIGMENTATION:
Formation of melanin is influenced by many different factors including genetics, certain medications, diet, and environmental factors. In today’s market there are many different types of skin-brightening ingredients that target the process of pigment formation via multiple modes of action. The term “tyrosinase inhibitor” has been used widely in the market to define a skin-brightening active. This term is true for certain active ingredients; however, not all skin-brightening ingredients directly interfere with the catalyzing of the tyrosinase enzyme. Tyrosinase is a copper-containing enzyme where copper and oxygen act as catalysts. Kojic acid is a specific ingredient that directly affects tyrosinase by chelating the copper within the enzyme-active site. The antagonist effect of kojic acid results in an inactive form of the tyrosinase enzyme that no longer allows for the production of melanin. As seen in Figure 6.2, the tyrosinase enzyme is a key component for melanin to be produced and should always be targeted in an effective skin-lightening formulation. Another area of focus is to look at other biochemical processes involved in melanogenesis. By looking at the “Pathway to Hyperpigmentation” we can analyze the different stages in which active ingredients can be targeted to more effectively control pigment production and produce the most visible changes in skin tone and appearance.
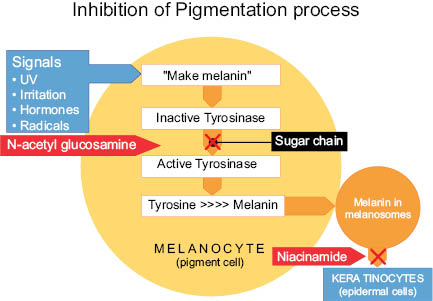
Figure 6.2. Inhibition of Pigmentation Process
- 1. One of the first things to occur in the skin along the pathway to hyperpigmentation is the generation of tyrosine, which is an amino acid naturally occurring in our bodies. Tyrosine is then converted to L-dopa by an enzyme called tyrosinase.
- 2. Tyrosinase catalyzes with L-dopa and converts it into dopaquinone.
- 3. Melanin is then created and put into our melanosomes.
- 4. Melanosomes are transferred into the keratinocytes, also known as our skin cells, which then continue on their journey up through the epidermis where they eventually are bio-converted to corneocytes and appear on the surface of the skin.
Besides the aforementioned pathways there are other ways to help suppress melanin formation. Melanogenesis is a complex process of oxidative stages in which topical antioxidants are useful to combat hyperpigmentation. Many antioxidants have skin-brightening properties that also provide multifunctional benefits. Antioxidants help to reduce hyperpigmentation by scavenging free radicals that can be a precursor to hyperpigmentation. Another example is MSH, melanin-stimulating hormone (melanotropin), which can be inhibited by certain ingredients. (1)
A variety of other avenues are used to increase cell turnover, which will provide skin-brightening results, as well as remove pigment from the surface for an instantly brighter glow. Alpha Hydroxy Acids (AHA) and kertolytic enzymes, such as papaya and bromalein, help to exfoliate surface stratum corneum cells to promote brighter, more even-tone complexion.Finally, the importance of using a UV absorber is crucial in order to see results and maintain those results from skin-lightening actives. Hyperpigmentation due to UV exposure can be addressed by using preventative measures, which include applying a physical/chemical broad-spectrum sunscreen regularly when exposed to UV radiation. The use of broad-spectrum photo-stable UVA/UVB sunscreens and protective clothing are one of the best ways to enjoy sun exposure while minimizing the deleterious effects of UV aggression on the skin. There are a number of companies who have woven UV absorbent fibers into protective clothing to prevent sun exposure. These items can be wide-brimmed hats, long-sleeved shirts, pants that when coupled with the use of UV-protectant sunglasses all help to protect the skin for extended times outdoors. The best commonsense photo-protective advice is to take cover in the shade when the sun’s rays are strongest between 10 a.m. and 4 p.m. Seek cover indoors or at least in a shaded spot during those hours when the UV index is at its most intense. Even though sun protection does not directly interfere with the pigment formation process, it helps to block UV radiation and limit post-sun exposure erythema, which are key triggers for melanocytes to produce melanin. The technological advances described above are quite important for formulators of sun-protection products since they describe the skin hyperpigment formation process and how we can target different pathways to inhibit it.
6.2.5 FORMULATING INGREDIENTS’A PLETHORA OF RAW
MATERIALS AND HOW THEY COME INTO PLAY
Actiwhite® PW LS 9860: Asynergistic skin-lightening complex based on pea extract and a sugar ester. This innovative mechanism has dual mode of action for optimal skin-lightening effect. The material does not affect the color of the formulation, has excellent compatibility and stability, and can be formulated in a broad pH range, even in combination with AHAs. This active component contains a high capacity to decrease melanogenesis. It works to decrease tyrosinase activity and reduce melanosomes from maturing.
Supplier: BASF Personal Care Ingredients
Arbutin: A potent antioxidant that is an extract from the bearberry plant. Arbutin works by inhibiting the activity of the tyrosinase enzyme to reduce hyperpigmentation and protects the skin against free radical damage. Arbutin suppresses the activity of tyrosinase enzyme and prevents melanosome from forming. Arbutin is chemically similar to hydroquinone.
Bearberry (Uva ursi) Extract: This plant leaf extract contains arbutin and methyl arbutin, both hydroquinone derivatives with skin-whitening properties.
β-White™ (Oligopeptide-68): An encapsulated peptide that mimics the signaling protein of the tyrosinase enzyme to reduce the amount of signaling proteins that activate tyrosinase to produce melanin. According to Unipex Innovations, an in vitro comparative study showed that β-White induced significant skin-lightening effects on 23 Asian volunteers with at least one hyperpigmented spot after four weeks.
Supplier: Lucas Meyer Cosmetics
Chromabright™ (Dimethylmethoxy Chromanyl Palmitate): An advanced engineered amino acid that fights against photo-aging. Chromabright reduces dopaquinone so that it cannot convert into DOPA, therefore inhibiting another pathway to melanin formation.
Supplier: Lipotec
Ethyl Ascorbic Acid: A novel vitamin C derivative with excellent whitening, anti-oxidation, radicals-scavenging and collagen-increasing effect. Its stability is superior to other ascorbic acid derivatives. It is widely used in skin care products, especially desirable for whitening and anti-ageing products.
Supplier: EME Ltd.
Glycolic Acid: Known as one of the most-used alpha hydroxy acids in the industry. Glycolic acid is naturally derived from sugar cane. It helps to speed up cell turnover and removes pigmentation from the outer layers of the skin to instantly brighten skin.
Hexylresorcinol: A widely known ingredient that has been used in the food industry for preservation and in oral and topical applications. Hexylresorcinol has antibacterial properties, and inhibits the tyrosinase enzyme to give a brighter, more even skin tone.(Symwhite: Symrise)
Hydroquinone: Hydroquinone is a very well-known ingredient to bleach the skin. Working through a variety of mechanisms, this ingredient reduces the formation of melanosomes and even induces melanocyte cytotoxicity. Hydroquinone is the only ingredient approved by the U.S. FDA for skin-whitening claims.
Kojic Acid: Derived from mushrooms and other species of fungus. This form of kojic acid is water soluble and inhibits tyrosinase enzyme by blocking copper from catalyzing with tyrosinase, which results in an inactive enzyme. This effective brightening agent also provides antibacterial properties.
Kojic Acid Dipalmitate: Derived from mushrooms and other species of fungus. This form of kojic acid is oil soluble and inhibits tyrosinase by chelating the copper contained within the tyrosinase enzyme resulting in a deactivated enzyme. This effective brightening agent also provides antibacterial properties. Compared to kojic acid, it offers improved efficacy and stability, and is easier to make with water-based formulations.
Esterases in the skin hydrolyze the ester bond, cleaving the fatty chain off kojic DP and allowing bioavailable KA to be liberated. (2)
Lactic Acid: A well-known alpha hydroxy acid that contains skin-brightening properties. Mostly derived from sour milk and sugars, this active exfoliates to instantly brighten skin and suppresses tyrosinase.
Licorice Root: This well-known extract inhibits the tyrosinase enzyme without causing cytotoxicity. This material also reduces UV-induced hyperpigmentation and inflammation in the skin, which are precursors to hyperpigmentation.
Lumiskin: Made up of triglycerides, this active ingredient acts as a competitive antagonist to the tyrosinase enzyme and suppresses the tyrosinase activity. Lumiskin inhibits calcium flow into the cells and thus reduces tyrosinase activity (stabilizes it in its inactive form) and melanogenesis.
Supplier: Croda
Lumisphere™: This complex combines Diacetyl Boldine and manganese titanium dioxide (TiO2-Mn) to instantly and gradually brighten the skin. Lumisphere, the Diacetyl Boldine (DAB) stabilizes tyrosinase in its inactive form. It also contains TiO2Mn, which can absorb UVA and UVB and neutralizes free radicals, resulting in a more even skin tone.
Supplier: Croda
Magnesium Ascorbyl Phosphate: Is a stable water-soluble derivative of vitamin C. It is an excellent antioxidant, stimulates collagen production, and is well known for its melanin-inhibiting properties.
Supplier: Caribbean Natural Products Inc.
The bioconversion needed to liberate the active form L-ascorbic acid phosphatase enzymes within the skin change this pro-form into bioavailable ascorbic acid.
References:
Mia Campos et al.: Ascorbic Acid and Its Derivatives in Cosmetic Formulations, Cosmetics and Toiletries, 11,59-62 (2000)
Melanostatine®-5: A skin-brightening peptide that is created in a lab to also inhibit the alpha-melanocyte-stimulating hormone (MSH). This active ingredient does not allow the hormone to signal the melanocyte to produce melanin, thus preventing and suppressing hyperpigmentation.
Supplier: Lucas Meyer (aka Unipex)
Meiritage: Combination of plant extracts used in Traditional Chinese Medicine: Atractylodes macrocephalae, Bupleurum falcatum, and Astragalus membranaceus roots, to prevent wrinkles and even out the complexion.
Supplier: Croda/Sederma
Mulberry (Morus bombycis): The root and bark extracts of the mulberry plant might also play a role in inhibiting the tyrosinase enzyme that converts tyrosine into melanin’s precursors. Mulberry contains arbutin, which inhibits melanin production.
O.D.A. White: Octadecenedioic acid is obtained by a biofermentation process from natural and vegetable oleic acid. O.D.A. White helps to inhibit the entire pathway of melanin synthesis from the nucleus of the melanocyte by reducing levels of tyrosinase mRNA. This active ingredient treats all types of pigmentation, even ethnic skin. The use of antioxidants in combination with ODA White is recommended.
Supplier: Croda
Phytic Acid: Most commonly found in grains, bran, and seeds. Phytic acid has been used as an antioxidant for years in the food industry. It works via a chelation mode of action by blocking the copper contained within the tyrosinase enzyme from catalyzing to form melanin.
Regu®-Fade: A nature identical trans-resveratrol ingredient based on a biofermentation that increases cell longevity and reduces inflammation. Resveratrol is made up of a polyphenol and has strong antioxidant properties to protect skin against free radicals and environmental damages. REGU®-FADE helps reduce the appearance of skin pigmentation as demonstrated in clinical and in vitro studies, resulting in noticeably brighter, younger-looking skin.
Supplier: DSM Nutritional Products, Inc.
Sepiwhite MSH (Undecylenoyl Phenylalanine): A highly advanced engineered active ingredient that inhibits the alpha-melanocyte-stimulating hormone (MSH).
Supplier: Seppic
Tetrahexyldecyl Ascorbate: Most commonly derived from citrus components. Advancements of vitamin C have been engineered to contain multiple benefits with greater efficacy and stability than L-Ascorbic Acid alone. Tetrahexyldecyl Ascorbate works as an antioxidant in the skin that also encourages brighter skin tone. This engineered form allows for better penetration into the skin and even suppresses melanogenesis up to 80% by inhibiting tyrosinase enzyme.
Tyrostat™ (rurnex occidentalis extract): Derived from plants native to Canada. Extracts of this plant have strong effect to inhibit the tyrosinase enzyme, resulting in a more even skin tone. Unipex screens the plant material for tyrosinase activity before it enters the extraction process to guarantee optimal activity. In vitro and clinical data have demonstrated that Tyrostat™ safely reduces skin pigmentation and erythema for a more uniform complexion. It also has outstanding results in the reduction of the appearance of age spots.
Supplier: Lucas Meyer Cosmetics
WONDERLIGHT™: An active ingredient of plant origin (hop cone), a regulator of the pigment disorders by inhibiting the cytokine GM-CSF (activator of melanogenesis).
Supplier: Croda/Sederma
Vitamin A: Also known as Retinol, is a multifunctional active ingredient that promotes youthful and even-toned skin. Vitamin A inhibits tyrosinase activity and reduces the amounts of melanosomes that are produced. This ingredient will also help to increase cell turnover and increase penetration of skin-brightening actives into the skin. When using a product containing Vitamin A and or its derivatives, it is mandatory to use in the evening only and accompany it with a broad-spectrum UVA/UVB SPF 30 during the day.
It is clear from the extensive array of the skin-whitening ingredients described above that formulators have great flexibility in selecting optimal agents and combinations of those agents to formulate products that address the global interest in skin whitening and brightening.
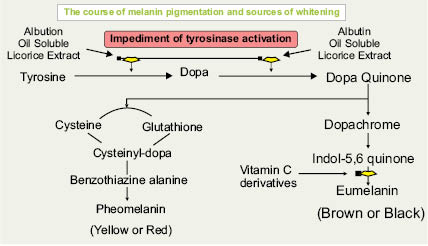
Figure 6.2.1
6.2.6 FORMULATIONS FOR INDIVIDUAL SKIN CONDITIONS
Whitening Cream
Ingredient Listing: Aqua (Water), Glycerin, Isopropyl Palmitate, Cetyl Alcohol, Stearyl Alcohol, Cetearyl Alcohol, Alpha Arbutin, Butylene Glycol, Kojic Dipalmitate, Dicetyl Phosphate, Ceteth-10 Phosphate, Hydrogenated Lecithin, Sodium Oleate, Oligopeptide-68, Glycyrrhiza Glabra (Licorice) Root Extract, Dimethylmethoxy Chromanyl Palmitate (Chromabright Proposed INCI), Sodium Metabisulfite, Sodium Sulfite, Phenoxyethanol, Sodium Ascorbyl Phosphate, Hexylresorcinol, Potassium Sorbate, Sodium Benzoate, Xanthan gum, Disodium EDTA
PHASE INGREDIENT/SUPPLIER

| Phase A | wt/% |
| Cetearyl Alcohol (and) Ceteareth 18 Symrise (2/014200 Dragowax S.E.) | 8.00 |
| Cetearyl Octanoate (and) Isopropyl Myristate Symrise | |
| (2/066210 PCL Liquid) | 3.00 |
| Mineral Oil Exxon Mobil (Marcol 82) | 5.00 |
| Octyl Octanoate Symrise (2/044115 Dragoxat EH) | 1.00 |
| Dimethicone Dow corning (200/350 cs) | 1.00 |
| Phase B | |
| Deionized Water | 75.45 |
| Xanthan Gum (Keltrol F) | 0.10 |
| Pentylene Glycol Symrise (2/016020 Hydrolite-5) | 3.00 |
| Glycerin BP | 1.20 |
| Phenoxyethanol (and) Methylparaben (and) Ethylparaben (and) | |
| Isobutylparaben (and) Propylparaben (and) Butylparaben | |
| Symrise (2/060140 Dragocide Liquid) | 0.80 |
| Sodium Hydroxide 1% Solution | 0.10 |
| C Tyrostat™-11 Unipex innovations | 1.00 |
| D Fragrance O/H 64583 The Vert Symrise | 0.30 |
PROCEDURE:
- 1. Disperse the Keltrol F in water.
- 2. Heat Phase A and B separately to 80°C.
- 3. Add Phase B into A under vigorous stirring and homogenize.
- 4. Allow batch to cool to 45°C and add Tyrostat-11 and homogenize.
- 5. Add the fragrance at 35°C.
Skin-Brightening & Anti-Aging Moisturizing Lotion with Resveratrol
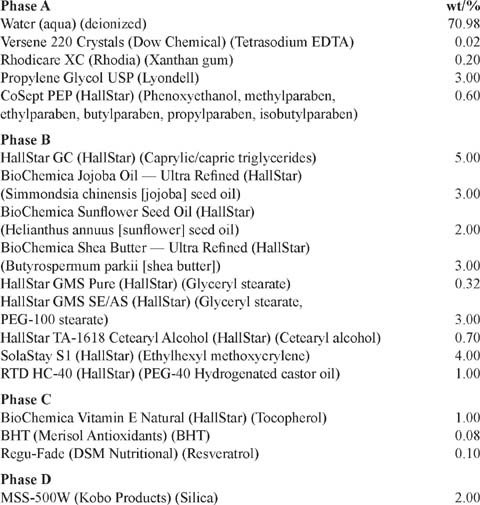
PROCEDURE:
To a suitable vessel equipped with mixing, heating, and cooling capabilities, add phase A and start mixing. To a second-phase vessel equipped with heating and cooling capabilities, charge phase B. Mix phases A/B while heating to 70°–75°C. When phases A/B are at 70°–75°C, slowly add phase B to phase A while maintaining temperature and adequate mixing. Homogenize for 10 minutes and then resume mixing and start cooling. At 122°F (50°C) or lower, add phase C and D; mix well while recirculating through a pump/mill combination. Cool to ambient temperature and then add water as needed to bring to full batch mass. When again uniform, stop mixing, perform final quality-assurance checks, and package product.
PROPERTIES:
(25°C): Appearance—Light yellow lotion; viscosity (RV, T-D, 5, 20 & 100 rpm, cP)—10800, 3500 & 1120; pH—6.0.
Reference: The HallStar Company
Skin-Lightening Cream Gel with Alpha-Arbutin and DISMUTIN®-PF

PROCEDURE:
- 1. Mix phase A together. Add phase B to phase A, mix until homogeneous.
- 2. Mix together phase C.
- 3. Premix phase D ingredients and add to phase C.
- 4. Add phase C/D to phase A/B. Homogenize to obtain a homogenous cream gel.
Reference: Centerchem, Inc.
High Protection SPF 30 Sunscreen with Skin-Lightening Properties
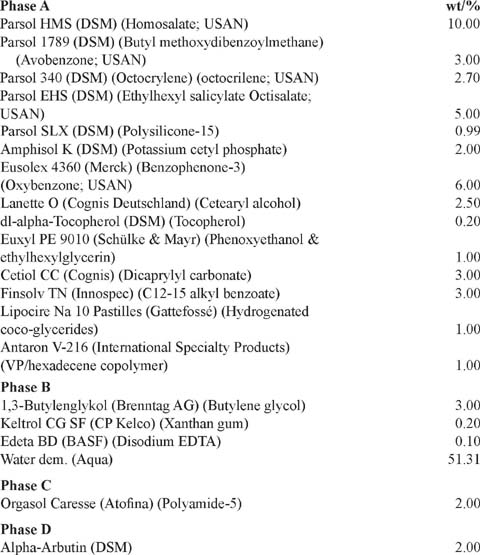
PROCEDURE:
Heat phase A to 85°C while stirring. Heat phase B to 80°C and add to phase A while stirring and homogenizing the emulsion. Cool down the emulsion to 40°C, add phase C, and homogenize again.
PROPERTIES:
pH—5.56 ;Viscosity—14600 cps (Brookfield, RV4,10 rpm); SPF in vivo—34.0 In vitro UVAPF (Colipa 2007)—1.7; PA—UVAPF/SPF (30 labeled); >0.33—0.39; PFA: Critical Wavelength—376.0.
Reference: DSM Nutritional Products
Skin-Brightening Cream with Chromabright MFF

PRODEDURE:
Heat phase B to 75°C. Slowly add carbomer to phase A water and mix until uniform. Begin heating to 75°C. Add remaining phase A ingredients and mix until uniform. When both phases are at 75°C, add phase B to phase A under moderate mixing. Mix for 10 minutes. Begin cool down. At 50°C, add phase C as a solution. Mix until uniform. At 35°C, add phase D ingredients. Mix until uniform.
Reference: Centerchem, Inc.,
Age Spot Treatment Cream

PROCEDURE:
Heat phase A up to 85°C; and also heat phase B up to 85°C. When both have the same temperature, add phase B to phase A while homogenizing intensively. Cool down the product to 35°C while stirring. Now add phase C and homogenize intensively again. It is generally recommended to use vacuum while producing the emulsion.
PROPERTIES:
pH—7.19; Viscosity—52800 cps (Brookfield, RV 6, 10 rpm).
Reference: DSM Nutritional Products
Brightening Lemon Under-Eye Crème
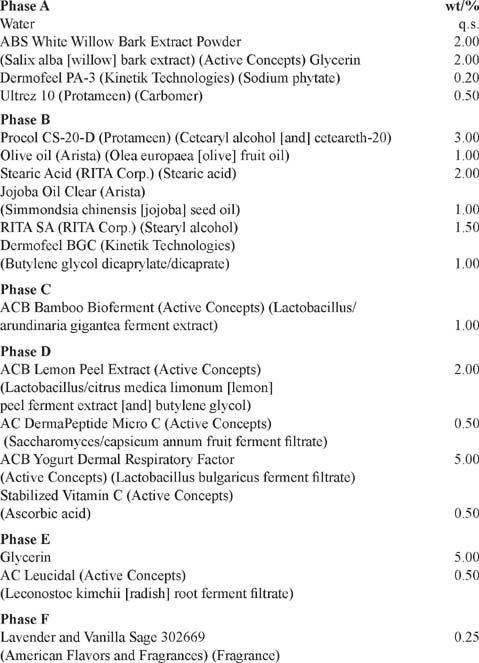
PROCEDURE:
Phase A: Charge water into main beaker and begin propeller mixing. A vortex should form. Begin heating to 75°C. Sift in ABS white willow bark powder extract. Charge glycerin and dermofeel PA-3. Sift in Ultrez 10.
Phase B: In a separate container, blend ingredients and heat to 80°C. Once temperatures have been reached, add to main. Maintain temperature of 78°C and continue mixing for 15 minutes.
Phase C: Remove heat. Add at 60°C.
Phase D: Add each at 45°C. Slowly sift in stabilized vitamin C. Pre-blend and add to main.
Reference: Active Concepts
Skin-Lightening Gel
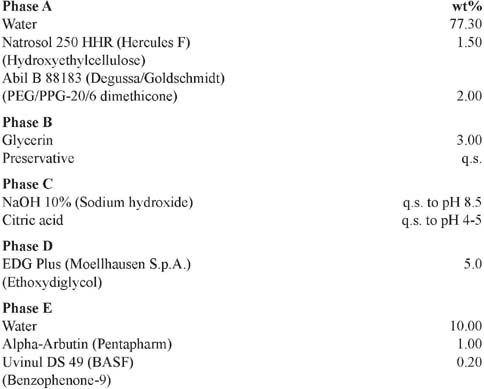
PROCEDURE:
Disperse Natrosol 250 HHR in water, then add Abil B 88183. Mix phase B, then add it to phase A. Adjust the pH to approx. 8.5 with NaOH 10% to obtain a clear gel. Then adjust the pH to approx. 4.0–5.0 with citric acid. Add phase D under stirring. Add phase E and mix to obtain a homogeneous gel.
Reference: Centerchem, Inc.
Skin-Lightening Cream for Age Spots

PROCEDURE:
Heat the ingredients of fatty phase A to 70°C. Heat the ingredients of water phase B to 75°C. While stirring add phase B to phase A, cool to 50°C, homogenize and cool to 30°C. Then add phase C and stir cold. Finally, incorporate phases D and E one after the other and adjust the pH to 4.5.
Reference: Centerchem, Inc.

PROCEDURE:
Place water and Hydrolite-5 in a mixer; add Sericite FSE and Tego Cosmo C 250 while stirring. Disperse by homogenizing briefly. Add Aerosil R 812 S VV 60 while homogenizing and homogenize for another 10 minutes. Check for complete dispersion. Add dimethicone 20cps, Abil B 8843 and Liquipar Oil while stirring and stir for another two to three minutes. Add Covagel while stirring and stir for another 15 minutes.
Reference: Degussa Corp. Business Line Aerosil
6.2.7 CLAIMS/REGULATIONS IN USA
Skin-Lightening Claims for USA:
Definition of a skin-bleaching active ingredient, according to the U.S. Food & Drug Association (FDA), is an agent designed to bleach or otherwise lighten limited areas of hyperpigmented skin through the suppression of melanin pigment formation within skin cells.
Hydroquinone is the only skin-bleaching active ingredient recognized by the FDA. The active ingredient and its concentration in the product are as follows for OTC: Hydroquinone 1.5 to 2.0 percent. Any increased concentration over 2 percent is considered a prescription that needs to be prescribed by a doctor.
Label Claims:
Hydroquinone may be combined with any generally recognized safe and effective sunscreen active ingredient, provided that the product is labeled accordingly.
The labeling of the product must contain the established name of the drug. It should identify the product as a skin-bleaching agent, skin lightener, or skin bleaching, e.g., lotion, cream, gel, etc. The labeling of the product contains a statement of the indications that is limited to the following phrases: “For the gradual fading or lightening of dark discolorations/spots/areas/freckles/age spots.” The label should also contain the appropriate warnings identified by the FDA.
For skin-lightening products that do not contain Hydroquinone, which are considered natural alternatives to hydroquinone, refrain from making any of the above label claims. These products contain other skin-brightening actives like kojic acid, brightening peptides, and licorice root extract. A common term used to describe these alternatives is “skin brightening,” or you can say it helps to promote a more even skin tone. Only skin-lightening products that contain Hydroquinone can make skin-lightening claims because Hydroquinone is the only active ingredient that is actually recognized by the FDA as a skin-bleaching agent.
REFERENCES
1. Schallreuter KU, Moore J, Tobin DJ, Gibbons NJ, Marshall HS, Jenner T, et al. alpha-MSH can control the essential cofactor 6-tetrahydrobiopterin in melanogenesis. Ann NY Acad Sci. 1999; 885: 329–41.)
2. Sarmad Al-Edresi et al., In-vitro and in-vivo evaluation of a photo-protective kojic dipalmitate loaded into nano-creams. Asian Journal of Pharmaceutical Sciences 2010, 5 (6): 251–265. Supplier: Caribbean Natural Products Inc.
Author
Charles Warren
Sunscreens (for body)
Sunshine contains both UV A and UV B rays, which are harmful to the living cells in the skin. UV B radiation is the cause of sunburn ranging from cellular damage to painful skin reddening and in some cases blistering and peeling. UV A radiation is related to the degradation of the cells and destruction of collagen and elastin cells leading to premature aging of the skin with all of the attendant negative effects—wrinkling, leathery skin appearance, etc. Sunscreens are chemicals that absorb the UV radiation and prevent the radiation from reaching the cells within the skin.
In the United States, sunscreens are regulated under an OTC monograph that dictates allowable sunscreens and levels, testing requirements, claims, labeling requirements, etc. Other countries throughout the world have their own regulations related to allowable sunscreens, levels, claims, label requirements, etc., and all of these requirements are not consistent. Sunscreens, levels, and claims allowed in one country may not be allowed in another. Similarly, countries may have different required testing for claims, stability, safety, etc. Formulators should consult the appropriate regulatory/legal groups prior to initiating formulation to determine what is and is not allowed in the market(s) of interest, what testing (functionality, analytical, stability, safety, etc.) may be required and any special registrations or preapprovals needed.
Sunscreens for the body are usually found in the following forms: cream/lotion, nonpressurized pump spray, or pressurized aerosol spray. The sunscreen ingredients for these products are chosen to: a) deliver the desired SPF rating; and b) be stable in the base formula for the desired form. The combination of and concentration of the individual sunscreens are what provide the desired SPF rating. Commonly employed sunscreens are: avobenzone, octisalate, oxybenzone, homosalate, octocrylene, titanium dioxide, and zinc oxide (the latter two actually blocking the UV radiation). The allowable levels of each sunscreen are indicated in the sunscreen monograph. Additional sunscreens are also included in the monograph. Suppliers of the various sunscreens can be very helpful in suggesting appropriate combinations and levels to achieve a particular SPF rating.
The creams and lotions are generally oil-in-water emulsions, composed of a water phase including water, glycerin, a thickening polymer (e.g., carbomer or the copolymers, such as acrylates/octylacrylamide copolymer), glycerin, C12–15 alcohol benzoate, and so on. The oil phase can include fatty alcohols (e.g., cetyl alcohol), fatty acids (e.g., stearic acid), dimethicone, distearyldimonium chloride, cyclopentasiloxane, and so on. The emulsification system, usually incorporated in the oil phase, is commonly a mixture of ethoxylated fatty alcohols (e.g., ceteth-20), and so on. The emulsion must be aesthetically acceptable both from visual and tactile perspective and must be stable, ensuring the stability of the entire formula, sunscreens included, to pass the requirements for expiration-date testing and functional delivery of the claimed SPF. It is not unusual to try multiple emulsions and blends to develop an acceptable formula that remains stable with the sunscreens incorporated.
Both pump sprays and pressurized aerosol sprays have similar base formulas, the difference being the use of the propellants in the pressurized aerosol forms. These forms are usually hydro-alcoholic solutions of silicones (e.g., dimethicone, cyclopentasiloxane), solvents/conditioners (e.g., glycerin), and other materials to assist in the dispensing of the sunscreens and provide aesthetically pleasing skin feel and uniform spreading of the sunscreen when applied. Propellants employed in the pressurized aerosol sprays include butane, isobutane, propane, and hydrofluorocarbon 152a, for VOC regulation.
Self-Tanning Lotions
During the late 90s and after, self-tanning lotion/sprays became popular. These formulas all rely on the chemical dihydroxy acetone (1,3 –hydrox-2-propanone). DHA reacts with the proteins in the skin to form a brown color approximately 1–2 hours after application. The concentration of the DHA (2–12%) dictates the darkness of the tan. The resultant “tan” is a purely visual, cosmetic effect, providing no UV protection to the skin as does the natural formation of melanin in an unaided exposure to sun. However, the development of a “tan” is accomplished without exposure to the other harmful effects of UVA and UVB radiation.
DHA can be formulated into creams/lotions (oil-in-water) emulsions and sprays (nonpressurized and pressurized). Formulas for these forms are similar to formulas already discussed in Hand and Body Lotions and Sunscreens. The aesthetics of the cream/lotion, the skin feel during and after application, and the development of the desired shade are the issues of importance to the formulator. Incorporation of erythrulose in the formula can impart depth and richness to the final shade obtained.
Author
Charles Warren
The most significant difference between these two subcategories is that an A/P is an over-the-counter drug while a deodorant is purely a cosmetic product. While the formulations may be very similar in nature, the OTC drug requirements are significantly different.
Both of these product subcategories are found in similar forms. The most common forms are: stick, pressurized aerosol spray, liquid/lotion/cream, roll-on, and powder spray. These forms also come under the regulation of maximum allowable VOCs, which can vary from state to state. Prior to initiating formulation, regulatory or legal groups should be consulted regarding the applicable regulations that will impact the final formula.
Perspiration is the body’s natural mechanism for cooling. Sweat, particularly in the armpit area, pools and allows bacteria to grow (warm, moist, undisturbed area). This accumulation of bacteria leads to the characteristic negative odor associated with the sweat. Deodorants are applied to allow the body to sweat but mask the unpleasant odor. Some deodorants contain materials to actually kill the bacteria while making the malodor. Antiperspirants contain materials that actually stop the sweat from exuding from the pores by creating small, gel-like plugs. These products also provide masking for any malodors that may develop from low levels of sweat that manage to exude. Because the function of the antiperspirant is to interfere or interact with a bodily function, it falls under the purveyance of an over-the-counter drug (OTC) and is bound by the specific monograph related to antiperspirants. The monograph indicates which active ingredients are allowed, what levels they may be used at, claims that may be made, etc. General OTC requirements govern stability testing required, manufacturing site registration, labeling requirements, etc. Again, of formulating OTC products, consultation with regulatory/legal groups is highly recommended.
The most common active ingredients found for the OTC products are aluminum zirconium tetrachlorohydrate (commonly used in sticks) and aluminum chlorohydrate (commonly used in sprays and roll-ons).
The base formulations for sticks generally include ingredients such as cyclopentasiloxane, stearyl alcohol, dimethicone, talc, and hydrogenated castor oil. There are a large number of other ingredients that can be used to provide different variations of stick composition. Sprays generally contain ingredients such as cyclopentasiloxane, isopropyl myristate, dimethicones, and propellants such as propane/butane/isoubutane blends and hydrofluorocarbon 152a (for VOC compliance). Lotions and creams are generally oil-in-water emulsions that contain lipoidal materials such as stearyl alcohol and ethoxylated fatty alcohols as emulsifiers.
ACNE, OILY, AND AGING SKIN PRODUCT
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







