Fig. 25.1
(a) Preoperative. (b) Marking. (c) Post operative following volume correction lower eyelids and lateral nasal defect with stem cell enhanced fat graft
25.2 What Are Stem Cells? (Fig. 25.2)
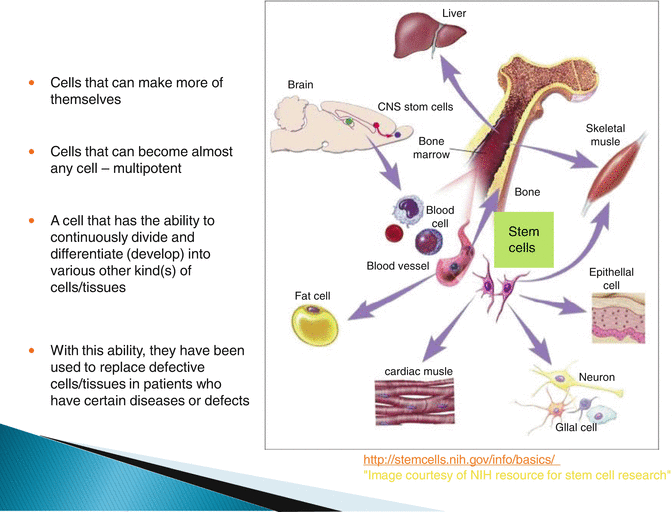
Fig. 25.2
What are stem cells?
Stem cells are those cells which have the ability in all multicelled organisms to divide, replicate and differentiate into specialised cell lines and are essential to the growth and development of the embryo and to the repair and renewal of the adult. A key issue is the matter of potency.
25.2.1 Pluripotent Stem Cells
Regarding stem cells the term pluripotent refers to cells that have the potential to differentiate into any of the three germ layers: the endoderm, the ectoderm or the mesoderm. The endoderm comprises the tube gut and the lungs. Ectoderm differentiates into the skin and the nervous system, and the mesoderm gives rise to the other tissue elements including the muscle, blood, bone, cartilage, connective tissues and adipose. Pluripotent cells are embryonic stem cells derived from the blastocyst.
25.2.2 Induced Pluripotent Cells
Induced pluripotent stem cells (IPSCs) are not really stem cells. They are adult cells which are forced to express a certain gene or transcription factor. They are artificially derived from non-pluripotent cells. These induced pluripotent cells are more ethically palatable than embryonic stem cells and express many similarities, including pluripotency, self-renewal, gene expression and morphology [1, 2].
It was initially thought that IPSCs would signal the end of the use of embryonic stem cells, due to their vast similarities. Further research with IPSCs indicated an increased risk of tumour growth which has hindered their use as a replacement for ESCs. The cost elements of production are also a critical factor.
25.2.3 Multipotent Cells
Multipotent cells describe cells that can potentially differentiate into a limited number of cells sharing a close relationship. Hematopoietic blood cells are a good example of multipotency. They are able to become any number of blood cell types but cannot become a non-blood cell type. Although multipotent cells are found in many human cell types, they are not found in all. Effectively this type of cell is an adult stem cell.
25.3 Stem Cell Sources
25.3.1 The Embryo
In a developing embryo the blastocyst stage is formed after about 5 days. As the name suggests it is a hollow sphere of cells comprised of two layers. The innermost layer ultimately becomes the embryo, and it is from here that the embryonic stem cells (ES) are isolated in the laboratory before the remainder of the blastocyst is destroyed. Once in the laboratory in nutrient-rich culture media, the cells are grown and perpetuated. At this stage these cells are pluripotent. Of course the necessary stimulating factors must be present to provide the impetus for differentiation. This is certainly a major challenge in science and the subject of much research. Cell regulations and technical and ethical considerations limit the use of either embryonic stem cells or induced pluripotent stem cells in clinical situations, even though these cells are, theoretically, highly beneficial.
25.3.2 Child Birth
Child birth offers a rare opportunity to harvest and store a person’s stem cells without any invasive procedures. It does not hold the same ethical concerns as embryonic stem cells but allows for the collection of pluripotent cells. A rich source of haematopoietic cells is the umbilical cord. It poses no risk for collection and is normally discarded. By collecting the blood that remains in the placenta and the attached umbilical cord, stem cells are able to be harvested and may possibly be used for autologous treatments later in the person’s life. As such it could be a viable alternative to bone marrow transplants. There have been a number of successfully treated health conditions with cells harvested in this way, including leukaemia, inherited diseases (of red blood cells, the immune system and certain metabolic abnormalities), lymphoma, myelodysplasia and severe aplastic anaemia. The umbilical cord also contains Wharton’s jelly which has a much higher concentration of multipotent stem cells, and it is here that you will find stem cells in their most primitive forms. It is a rich source of mesenchymal stem cells. There are several stem cell genes expressed in Wharton’s jelly, including telomerase. Wharton’s jelly holds potential as a source of adult stem cells as these primitive cells are able to be induced to differentiate into mature cell types including neurons [3].
The amnion and chorion make up another incredibly rich potential source of cell lines. These two tissues of course make up the placenta which at its basic is designed to facilitate growth of a foetus as well as provide protection against antigenic assault from the maternal and even the external environment. Amnion has been known for over 100 years as an effective wound dressing.
There is much more to be gained from investigation and trails into the potential uses of amniotic tissue. Amniocentesis during foetal development yields fluid and cells for a variety of testing and screening processes but also can be an additional source of multipotent mesenchymal stem cells. Access to umbilical cord contents and placental tissue is a once-in-a-lifetime event which at the moment we largely squander due to insufficient understanding of the potential. Although there is an increasing prevalence of cord blood and placental tissue banking, the science requires significantly more effort and input.
25.3.3 Adult Stem Cells
Adult stem cells are far less controversial than their embryonic counterparts and a more viable option. The capability to give rise to all the cell types of the organ from which they originate offers the potential to regenerate entire organs from a few cells. Adult stem cells are undifferentiated cells that are capable of self-renewal and can differentiate to yield specialised cell types of organ or tissue. Their primary role is the maintenance and repair of tissues and organs. There are different types of adult stem cells and often more than one type is found in any given source.
In many potential applications one has to consider the development of a cellular scaffold or support structure to provide the mechanical framework to regenerate a functioning organ from nests of stem cells. This scaffolding process itself is also replete with patent issues and IP restraints.
25.3.4 Mesenchymal Stem Cells
Mesenchymal stem cells (MSC) have been sourced most commonly from the bone marrow but have been isolated from the peripheral blood as well as umbilical cord blood and Wharton’s jelly, and more recently adipose tissue has been identified as a prolific source of MSC. They can also be found in the dental pulp of deciduous teeth. With such a source with such ease of collection, this could become a further option for harvest and banking of regenerative tissue. Mesenchymal stem cells are exclusive to the mesenchyme, they are derived from embryonic connective tissue formed in the mesoderm, and, being multipotent, they have the ability to differentiate forming adipocytes, cartilage, bone, muscle, skin and tendon. MSCs then have an enormous potential for therapeutic use in tissue repair [4].
Mesenchymal stem cells are morphologically characterised by a large, round nucleus and prominent nucleolus, contained within a long, thin cell body. They are able to maintain multipotency even during the process of self-renewal. Advances in research and development have allowed us to observe MSC’s capabilities to differentiate into multiple cell types and follow their progression into muscular, osteogenic, chondrogenic and adipogenic lineages as a repair and renewal function.
With regenerative medicine becoming a more exciting prospect for those patients who show a vested interest in maintaining their own health, the potential for autologous cell base therapies is exponential and mesenchymal stem cells seem to be the ideal stem cell population for practical regenerative medicine.
It is important to consider the terminology when discussing stem cells. Many cell lines share similar traits, but this should not be confused with them being similar cells. Each cell line should be classified in its own right. The mesoderm contains the mesenchyme which is embryonic connective tissue cells derived from the mesoderm; they are able to differentiate into haematopoietic cells and connective tissue. The connective tissues that form support structures house stromal cells. The term multipotent stromal cells has been proposed as a replacement term to encompass these cell lines. The term MSCs is often incorrectly used for both mesenchymal stem cells and multipotent stem cells. As you can see from the descriptions given above, this is incorrect and warrants clarification.
Adult stem cells are identifiable in many of the body tissues and organs; this includes the bone marrow, blood vessels, skin, teeth, liver, ovarian epithelium, testis, brain, skeletal muscle, gut and heart. It is thought that each tissue has a specific area in which they reside, referred to as a ‘stem cell niche’ which is a regulated microenvironment. This ‘niche’ has factors, which in embryonic stem cells will act to alter gene expression or promote differentiation and the induction of proliferation in a developing foetus.
In the human body, stem cells will often remain dormant or inactive, until an injury to tissue occurs, when the surrounding microenvironment will signal the stem cells to trigger to proliferation and aid in new tissue formation and repair.
Current evidence suggests that in many tissues stem cells are pericytes but perhaps not all pericytes are stem cells. Crisan et al. [5] stated that mesenchymal stem cells (MSCs), the archetypal multipotent progenitor cells derived in cultures of developed organs, are of unknown identity and native distribution. The authors have prospectively identified perivascular cells, principally pericytes, in multiple human organs including the skeletal muscle, pancreas, adipose tissue and placenta and on CD146, NG2 and PDGF-Rβ expression and absence of haematopoietic, endothelial and myogenic cell markers. Perivascular cells purified from skeletal muscle or non-muscle tissues were myogenic in culture and in vivo. Irrespective of their tissue origin, long-term cultured perivascular cells retained myogenicity; exhibited at the clonal level osteogenic, chondrogenic and adipogenic potentials; expressed MSC markers; and migrated in a culture model of chemotaxis. Expression of MSC markers was also detected at the surface of native, noncultured perivascular cells. Thus, blood vessel walls harbour a reserve of progenitor cells that may be integral to the origin of the elusive MSCs and other related adult stem cells.
Pericytes are the cells that makeup small blood vessels most outer layer. Stem cells may lie dormant, not dividing for extended time periods until activation occurs as a result of a tissue or organ needing to be maintained or repaired through injury or disease. Typically, the number of stem cells in each tissue is very small and their capacity to divide becomes restricted once removed from the body, making it difficult to generate large quantities of stem cells without manipulation through in vitro expansion. Scientists are constantly searching for better ways to grow these large quantities of adult stem cells in various culture media’s and produce cell types specific to the injury or disease to be treated. Examples of this would be the regeneration of bone from cells procured from bone marrow stroma, Type 1 diabetes being treated with insulin producing cells or damaged heart muscle being repaired with cardiac muscle cells following heart attack. Clearly in these circumstances the challenges of delivery to the affected site and implantation of functioning organ elements are significant.
25.4 Haematopoietic Stem Cells
Haematopoietic stem cells were the first stem cells to be identified. They are ultimately responsible for the constant renewal of blood and immune cells. Haematopoietic stem cells (HSC) are multipotent, allowing them to differentiate into specific types of cells. For example, HSCs found in the peripheral blood have the capability of forming all blood cells, including white blood cells, red blood cells and platelets. Haematopoietic stem cells are found in the peripheral blood and the bone marrow. Morphologically, they have similar characteristics to white blood cells in that they can be difficult to identify with shape and size alone, so it is generally cell surface proteins that are used to distinguish these cells.
25.5 Bone Marrow
The bone marrow contains at least 2 populations of stem cells, haemopoietic and bone marrow stromal cells or mesenchymal stem cells. A commonly held belief is that stem cell treatment is a new area of medicine, but those people would do well to be reminded that doctors realised decades ago that patients with bone marrow disorders would benefit from a bone marrow transplant. Bone marrow is essential to make the specialised blood cells that we need to survive [5]; successful transplants led to questions of how these haematopoietic cells could be used in different conditions and begun the interest in stem cells that we see today. Bone marrow stromal cells are ideally suited to patients who have undergone treatments or therapies that have destroyed their own body’s abilities to renew its own blood cells as these treatments often affect cells that divide rapidly – as in chemotherapy and radiation therapy. Bone marrow transplant is essentially a ‘stem cell’ transplant in that it allows the patient’s body the ability to make its own blood cells required to make oxygen, fight infection and prevent bleeding. The bone marrow is a rich source of haematopoietic stem cells with potential to treat many conditions, but harvesting these cells is invasive with considerable recovery time [6, 7].
25.6 Adipose Tissue
Adipose tissue has attracted interest as a proposed alternative to bone marrow due to the ease of collection, high yield and relative low patient downtime. We are able to harvest large amounts of adipose tissue and isolate cells. There are a number of techniques currently implemented in the isolation process, and the effectiveness of each is still debatable, and whilst products such as collagenase and lipase produce significantly higher cell numbers, it is somewhat more cumbersome and labour intensive. Quality issues however are superior in the authors’ opinion than the alternative ultrasonic emulsification methods. Most studies currently available concentrate on expanded cells, but in essence, the use of a stromal vascular fraction is a refined method of the autologous fat grafting widely used for around 15 years in common modern practice. From a safety perspective, fat grafting is fairly innocuous. But it is important to consider the various components of the whole lipoaspirate when considering clinical implications.
Adipose tissue is a rich source of mesenchymal stem cells, and after harvest and centrifugation, adipose separates into layers, one of which contains the cellular component. It is this layer that is commonly referred to as the stromal vascular fraction (SVF). These cells are morphologically similar to fibroblasts and subsequent to in vitro expansion show similar surface marker expression to bone marrow-derived stem cells. They have demonstrated the ability to differentiate into myogenic, adipogenic, chondrogenic and osteogenic lineages.
SVF has been found to contain endothelial precursor cells (EPC). In addition to MSCs and EPCs, SVF is also known to contain monocytes, macrophages, histiocytes, fibroblasts and a variety of growth factors. The macrophage component of SVF was initially thought to be attributed to chronic low-grade inflammation.
25.7 Harvesting Options
Autologous adult stem cells are readily accessible from three sources in humans.
25.7.1 Bone Marrow
The bone marrow requires harvesting via aspiration and involves drilling into bone. Aspiration of the bone marrow will generally be performed on the back of the hipbone or the posterior iliac crest and the donor is anaesthetised. This is the classic form of stem cell collection/donation. Doctors have been performing bone marrow transplants for over 40 years. For every 100,000 cells collected from the bone marrow, about 1 is a long-term, blood-forming stem cell; the other cells include blood progenitor cells, stromal cells, stromal stem cells, red blood cells and white blood cells. Retrieving stem cells via bone marrow aspiration is becoming a fading practice as modern medicine develops less invasive procedures to procure stem cells.
But ‘priming’ the patient by the administration of cytokines induces migration of stem cells from the bone marrow to the blood, greatly increasing numbers and making it safer for the patient (as opposed to the increased risks associated with bone marrow harvest) and the cell quality is better. Peripheral blood collections’ main drawback would be the haemolysis of the cells due to incorrect collection technique, whereas collection of bone marrow has a whole host of problems for cell quality as well as patient-related difficulties and post-collection pain.
25.7.2 Peripheral Blood
Peripheral blood cell harvest requires no anaesthesia or hospital stay and also yields better cells for transplantation. This refers to the quality of the harvest in that the number of viable cells is greater. In a clinical study by Dr Richard Childs, Acting Clinical Director of the National Heart, Lung, and Blood Institute’s (NHLBI) Division of Intramural Research (DIR), evidence suggests that cells harvested from peripheral blood contain up to twice as many haematopoietic stem cells (HSCs) than those from the bone marrow and engraft more quickly. White blood cell and platelet recovery in patients may be quicker and their immune response and clotting protection can improve several days faster than if they underwent a stem cell transplant with cells collected from the bone marrow. Stanford scientists have reported that these cells in patients who have underwent intensive chemotherapy following breast cancer show signs of swift engraftment and report fewer complications [8, 9].
A small number of progenitor and stem cells have been known to circulate in that bloodstream for decades. It is only in the last 10 years that research has been conducted that shows these cells can be coaxed to migrate to the blood from marrow in increased numbers. To trigger this response the donor must be injected a number of days prior to harvest with a cytokine. These cells can be collected intravenously and are filtered prior to returning the cells to the donor. This method yields a mixture of stem cells, progenitor cells and white blood cells of various maturity; of these cells only 5–20 % will be true HSCs [10].
25.7.3 Adipose Tissue
Adipose tissue (lipid cells) requires extraction by liposuction. In our modern society there seems a general abundance of available adipose tissue. Adipose tissue harvest yields the stromal vascular fraction (SVF). A sample of lipoaspirate after centrifugation will separate into a lipid portion, a fluid portion and the stromal vascular fraction (Fig. 25.3).
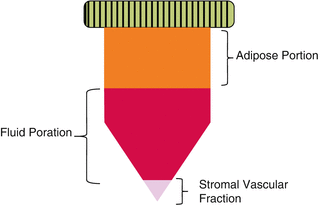

Fig. 25.3
Stromal vascular fraction obtained after the centrifugation of the lipoaspirate (en.wikipedia.org/wiki/Stromal_Vascular_Fraction. Accessed 10/1/13)
If the lipoaspirate is first altered by enzymatic action (collagenase or lipase) or alternatively by ultrasonic means, the resultant SVF portion will be greater than in the unmanipulated sample. There are debateable points about the ultimate viability of the cells obtained by different methods. What is clear however from the authors own practice is that delicate handling of the tissue sample from harvest to isolation of SVF will produce a better result. A ‘standard’ collection involves approximately 100 g of adipose tissue which can be expected to result in approximately 100 million cells or in other words one million cells per gram of fat. Most uses in clinical practice revolve around this volume and this cell count per application. The authors’ practices encompass cosmetic uses for enhanced fat grafting, skin repair as well as a variety of musculoskeletal applications most commonly arthropathy of the knees, ankles and hips.
Practice has shown the 100 million cell count as effective in these applications per joint injection as well as the cosmetic applications. Further, practice has shown that in joint injections, a series of injections (most commonly 3 or 4) involving a total of 100 million cells per joint provides a better long-term efficacy than a single injection of the same number of cells. It is presumed although not yet shown definitively that cosmetic uses may have a similar response. Storage issues are discussed below and of course are germane to success.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








