Fig. 56.1
Merkel cell carcinoma. An asymptomatic, firm, purple tumor on the face of an old patient
TMCC can be staged as follows:
Stage I – Primary tumors ≤2 cm, without evidence of regional lymph node involvement
Stage II – Primary tumors >2 (T2 or T3) or a primary tumor with invasion into the bone, muscle, fascia, or cartilage (T4)
Stage III – Any primary tumor with regional lymph node disease
Stage IV – Metastasis beyond the regional lymph nodes, regardless of the status of the primary tumor and regional nodes
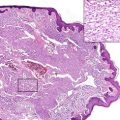
Pathology
MCC usually appears as a dermal nodule (Fig. 56.2), which frequently extends into the subcutaneous fatty tissue. The tumor cells are small, blue, round to oval, and of uniform size, with basophilic nucleus and minimal cytoplasm (Fig. 56.3). Nuclear membranes are distinct, the chromatin is finely dispersed, and nucleoli are usually inconspicuous; mitoses and apoptotic bodies are numerous (Fig. 56.4). Tumor cells may be focally spindled and rarely large, especially in recurrences after radiotherapy. Epidermotropism of tumor cells is uncommon, and in exceptional cases, the tumor cells are entirely limited to the epidermis. Ulceration of the epidermis occurs in a subset of cases. The papillary dermis and adnexa are usually spared.
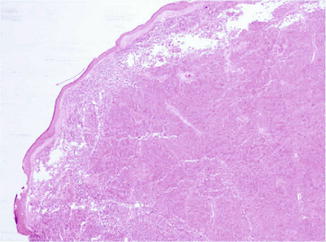
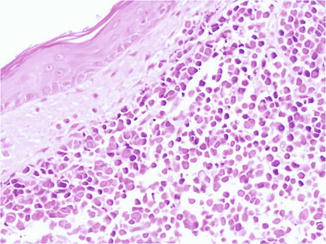
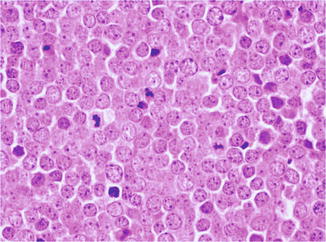

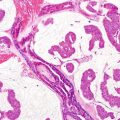
Fig. 56.2
Merkel cell carcinoma. A dermal bluish nodule made up of densely packed cells

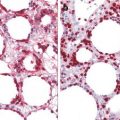
Fig. 56.3
Merkel cell carcinoma. The tumor cells are small, round to oval, and of uniform size, with basophilic nucleus and minimal cytoplasm

Fig. 56.4
Merkel cell carcinoma. Nuclear membranes are distinct, the chromatin is finely dispersed, and nucleoli are usually inconspicuous, while mitoses are numerous
Three patterns have been recognized: the trabecular cell type seen in 25 % of cases in which the cells are arranged in interconnected trabeculae separated by strands of stromal tissue; the intermediate cell type, observed in over 50 % of patients, in which large nest of cells without organoid architecture are recognized with a better prognosis compared to the other ones; and the small cell type, the least common one, where solid sheets and clusters of cells separated by abundant stroma with large areas of necrosis are seen. Additional pattern include: squamous, melanocytic, eccrine, leiomyosarcomatous, rhabdomyoblastic, and fibrosarcomatous differentiation.
Larger lesions may show angiolymphatic involvement and scattered or dense infiltrate of lymphocytes and sometimes plasma cells. Vascular proliferation is present in approximately 20 % of cases. The concurrence of MCC and squamous cell carcinoma or Bowen’s disease is also documented.
MCC shows epithelial and neuroendocrine differentiation. Tumor cells express low-molecular-weight cytokeratins (detectable by specific or broad-spectrum cytokeratins such as AE1/AE3, CAM5.2), epithelial membrane antigen, and the epithelial marker Ber-EP4. Positive staining for anti-cytokeratin 20 (CK20) is quite sensitive (Fig. 56.5a); in fact, CK20 is expressed by 97 % of MCC.
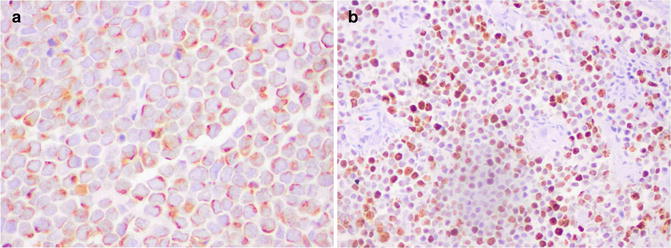

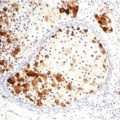
Fig. 56.5
Merkel cell carcinoma. (a) Tumor cells are positive for cytokeratin 20 with the typical paranuclear dot pattern, and (b) the Ki-67 proliferation index is very high
The staining pattern for low-molecular-weight cytokeratins and CK20 typically consists of paranuclear dots but may also be cap-like paranuclear or diffuse cytoplasmic staining. Markers of neuroendocrine differentiation include chromogranin, synaptophysin, neuron-specific enolase (NSE), bombesin, somatostatin, calcitonin, and gastrin. Merkel cell carcinoma also expresses CD117. The Ki67 proliferation index evaluated with Ki67 is high (Fig. 56.5b). Ultrastructural studies reveal the presence of dense core neurosecretory granules, tightly packed intermediate filaments, and desmosomes in the proliferating cells.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree