31 Melanoma
Synopsis
 The definitive diagnosis of melanoma is based on histologic analysis. Clinical features suggestive of malignancy include: asymmetry, border irregularity, color changes, diameter >0.6 cm, and evolving changes – the ABCDE criteria.
The definitive diagnosis of melanoma is based on histologic analysis. Clinical features suggestive of malignancy include: asymmetry, border irregularity, color changes, diameter >0.6 cm, and evolving changes – the ABCDE criteria.
 The four major histopathologic subtypes of melanoma are: lentigo maligna, superficial spreading, nodular, and acral lentiginous. Desmoplastic melanoma is a less common subtype of melanoma that lacks pigment and may demonstrate perineural invasion.
The four major histopathologic subtypes of melanoma are: lentigo maligna, superficial spreading, nodular, and acral lentiginous. Desmoplastic melanoma is a less common subtype of melanoma that lacks pigment and may demonstrate perineural invasion.
 Initial workup of the pigmented lesion should include excisional biopsy with a 1–2-mm margin of normal-appearing skin. If functional or cosmetic concerns prohibit removal of the entire lesion, incisional or punch biopsy may be performed.
Initial workup of the pigmented lesion should include excisional biopsy with a 1–2-mm margin of normal-appearing skin. If functional or cosmetic concerns prohibit removal of the entire lesion, incisional or punch biopsy may be performed.
 Histologic evaluation of the primary lesion must include: Breslow depth in millimeters, presence/absence of ulceration, mitotic rate per mm2, peripheral and deep margin status, and Clark level (especially for lesions ≤1 mm in depth).
Histologic evaluation of the primary lesion must include: Breslow depth in millimeters, presence/absence of ulceration, mitotic rate per mm2, peripheral and deep margin status, and Clark level (especially for lesions ≤1 mm in depth).
 Recommended excision margins are determined by Breslow depth. In situ melanoma requires a 0.5-cm margin of normal-appearing skin. For invasive melanoma, a 1-cm margin is recommended for lesions ≤1 mm in depth, a 1–2-cm margin is recommended for lesions 1.01–2.0 mm in depth (depending upon functional/cosmetic concerns), and a margin of at least 2.0 cm is recommended for lesions >2.0 mm in depth.
Recommended excision margins are determined by Breslow depth. In situ melanoma requires a 0.5-cm margin of normal-appearing skin. For invasive melanoma, a 1-cm margin is recommended for lesions ≤1 mm in depth, a 1–2-cm margin is recommended for lesions 1.01–2.0 mm in depth (depending upon functional/cosmetic concerns), and a margin of at least 2.0 cm is recommended for lesions >2.0 mm in depth.
 Subungual melanoma of the hand should be resected at the distal interphalangeal joint to preserve function.
Subungual melanoma of the hand should be resected at the distal interphalangeal joint to preserve function.
 Sentinel lymph node biopsy is offered to patients with melanomas >1 mm in thickness, and patients with thin melanomas (≤ 1 mm thick) that demonstrate high-risk features, including ulceration and/or high mitotic rate. The likelihood of detecting metastatic deposits in a sentinel lymph node biopsy increases with the thickness of the primary lesion.
Sentinel lymph node biopsy is offered to patients with melanomas >1 mm in thickness, and patients with thin melanomas (≤ 1 mm thick) that demonstrate high-risk features, including ulceration and/or high mitotic rate. The likelihood of detecting metastatic deposits in a sentinel lymph node biopsy increases with the thickness of the primary lesion.
 For patients with stage I and II disease, chest X-ray and liver function tests compose the recommended workup. Patients with regional (stage III) or systemic metastases (stage IV) should undergo a comprehensive staging workup that may include computed tomography scans with positron emission tomography imaging.
For patients with stage I and II disease, chest X-ray and liver function tests compose the recommended workup. Patients with regional (stage III) or systemic metastases (stage IV) should undergo a comprehensive staging workup that may include computed tomography scans with positron emission tomography imaging.
 Serum lactate dehydrogenase is used in the American Joint Committee on Cancer staging system as it portends a worse prognosis in patients with metastatic disease.
Serum lactate dehydrogenase is used in the American Joint Committee on Cancer staging system as it portends a worse prognosis in patients with metastatic disease.
 Patients with high-risk primary tumors or metastatic disease should be considered for adjuvant treatment with interferon-alpha or enrollment in a clinical trial.
Patients with high-risk primary tumors or metastatic disease should be considered for adjuvant treatment with interferon-alpha or enrollment in a clinical trial.
Introduction
Epidemiologic studies demonstrate that the incidence of melanoma has been increasing faster than that of any other cancer in the US.1 For the year 2010, in the US alone, 68 130 new melanoma cases were diagnosed and 8700 deaths were attributed to melanoma.2 While melanoma accounts for roughly 4% of all skin cancers, it is responsible for more than 77% of skin cancer deaths. Current estimates of the lifetime risk for developing invasive melanoma is 1 in 37 for white men and 1 in 56 for white women.2 Our understanding of melanoma continues to improve, and we can now differentiate low-risk from high-risk patients on the basis of multifactorial analyses from several series of large numbers of patients. However, despite our best attempts to understand the molecular basis of this disease, limited success has been demonstrated in terms of new medical treatments, and successful treatment of this disease relies heavily upon the surgeon.
Historical perspective
Although Hippocrates is credited with the first reported observation of what appears to be a melanoma, Handley3 attributes the first clinical case of malignant melanoma reported in England to Dr. William Norris. In 1820, Norris felt compelled to report this case because of the rapid spread of the tumor, which led to his patient’s death. He reported that, at the autopsy, every organ except the spleen and the bladder was riddled with “black specks.”4 The patient’s father had died of similar disease, and the patient’s brothers and his children had “many moles on various parts of their bodies.”
Handley had never himself treated a patient with a melanoma, but he made considerable observations after performing an autopsy on a 34-year-old woman who had died of disseminated metastatic melanoma. He was interested in the mechanisms of spread of melanoma. Halsted had already described the spread of breast cancer along the lymphatics surrounding the primary tumor.5,6 In his two hunterian lectures, Handley proposed that the primary spread of melanoma was through the lymphatics and not by blood vessels. In his first lecture, he described his autopsy findings: the 34-year-old woman previously had a primary melanoma excised from her right foot over the achilles tendon. At autopsy, Handley observed a large collection of tumor growths in the right groin and virtually every part of the body except the entire left leg (Fig. 31.1). Arguing incorrectly that every organ would have been involved if tumor spread were hematogenous, he stated that sparing of the spleen, bladder, stomach, and left leg could be explained by “lymphatic permeation.” Although Handley did not discount the concept of hematogenous spread, he believed that early dissemination was by lymphatics and that invasion of the blood stream occurred later “either by local infiltration of veins from concomitant permeated lymphatics, or by malignant cells carried into the blood along the thoracic duct from invaded lymphatic glands.”3
In his second lecture, Handley proposed that lymphatics were amenable to surgical removal with the primary melanoma. He based his treatment recommendations on histologic examination not of the primary site but of the regional groin recurrence. Indeed, Handley had confessed that “no opportunity of investigating the spread of permeation round a primary focus of melanotic growth has fallen to me.”7
Nevertheless, because the site of the groin recurrence had shown “permeation spreading centrifugally in the lymphatic plexus of the deep fascia and invading skin and muscle secondarily over a smaller area,” he advocated a wide radical resection for a primary melanoma (Fig. 31.2):
A circular incision should be made through the skin round the tumour … situated as a rule about an inch from the edge of the tumour, should be just deep enough to expose the subcutaneous fat. . . . The skin with a thin attached layer of subcutaneous fat is now to be separated from the deeper structures for about two inches in all directions round the skin incision…. Finally, the whole mass with the growth at its centre is removed by scooping out with a knife a circular area of the muscle immediately subjacent to the growth.7
In the year after Handley’s lecture, Pringle8 described 3 patients with melanoma whom he had treated during the previous 10 years. However, Pringle further advocated removal of all subcutaneous tissue, including the lymphatics, between the primary site and the regional nodes together with the specimen. This was the first paper to describe the technique of an in-continuity dissection. He reported that one of his patients – a young woman who was alive 9 years after surgery at the time of the report – had moved to Canada, married, and had children. Pringle concluded in his report that “all that is removed should be in one continuous strip as far as possible.”
Nowadays the improved operation for breast cancer produces prolonged or permanent immunity in about 50 percent of cases. And upon the evidence I have laid before you I venture to predict that the application of more thorough and scientific methods to the surgery of cutaneous melanomata will produce a corresponding, though perhaps a smaller, improvement in the results of operation.7
However, the methods of treating melanoma changed very little during the first half of the 20th century. The various reports of cure rates after aggressive surgical treatment of melanoma remained the same: a better prognosis if the patient did not have clinically palpable nodes (80% 5-year cure rate for stage I disease) than if the nodes were palpable (40% cure rate for stage II disease).9
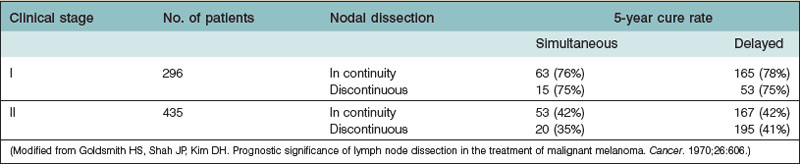
Subsequently, surgeons gained some information on prognostic factors of this tumor. Within each clinical stage, the prognosis is improved by the pathologic findings of no evidence of metastatic tumor cells on microscopic examination of the removed lymph nodes. However, the advantage of in-continuity removal of all tissues between the primary site and the regional lymph nodes, as advocated by Pringle8 and as widely employed for decades, was challenged by Goldsmith et al.,9 who demonstrated no difference between this technique and that of discontinuous dissection of the primary tumor and the lymph nodes (Table 31.1). They, too, found no difference between immediate and delayed lymphadenectomy.
Finally, the issue regarding excision of underlying muscle and fascia was evaluated and resolved. Olsen10 reviewed a series of 67 patients treated for melanoma in Denmark between 1949 and 1957. She found a 45% incidence of subsequent regional nodal metastases among the 31 patients who had the fascia removed during excision of the primary tumor and a 14% incidence of metastases among the 36 patients in whom this fascia was left intact. Because the patients who underwent a fasciectomy may have had more aggressive tumors, Olsen reviewed the next 51 patients treated from 1958 to 1961 who did not undergo excision of the fascia and observed only a 10% incidence of regional metastases. Kenady et al.11 reviewed their data of 202 patients treated at the MD Anderson Hospital, Houston, from 1961 to 1974 and found that local recurrence, regional nodal recurrence, distant metastases, and survival were not statistically different between the 107 patients who had the fascia excised and the 95 patients who did not. There appears to be no indication for removal of underlying muscle fascia unless the fascia becomes involved by contiguous tumor growth.
Clinical evaluation
Clinical diagnosis
Junctional nevus
Junctional nevi are small flat lesions that first appear after birth and are smooth, nonpalpable, and light to dark brown or black (Fig. 31.3A). They are called junctional because the nevus cells are located at the interface of the epidermis and dermis. As the person develops and matures, the nevus cells grow and push into the dermis to develop into the common adult intradermal nevus.
Compound nevus
As the nevus matures, the central portion pushes into the dermis, causing this central portion to elevate and appear thicker (Fig. 31.3B). This nevus is called compound because the central portion is intradermal and thick, whereas the periphery is still junctional and flat. Compound nevi often are seen during adolescence, and the changes in such moles may cause concern to the patient, family, or primary care physician.
Intradermal nevus
The intradermal nevus is the common adult mole of the face or trunk that is elevated because of the maturation and proliferation of the nevus in the dermis, which now pushes up the overlying epidermis (Fig. 31.3C). It may be light or dark, usually is elevated, and may be sessile or pedunculated.
Blue nevus
Most nevi appear brown or black because the melanin is superficial and absorbs light. When the nevus contains melanin that is located more deeply, blue wavelengths of light pass through the less pigmented epidermis and are reflected back to the eye as a blue nevus (Fig. 31.3D).
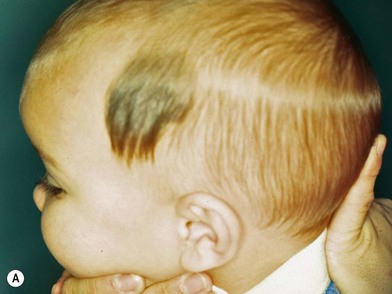
Congenital nevus
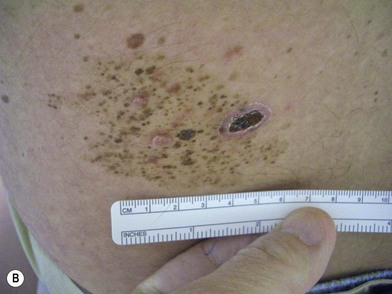
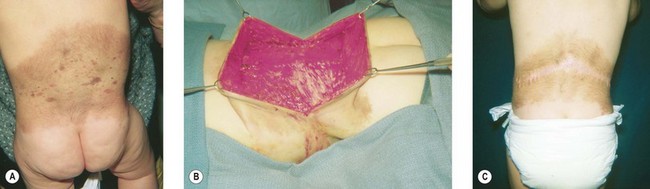
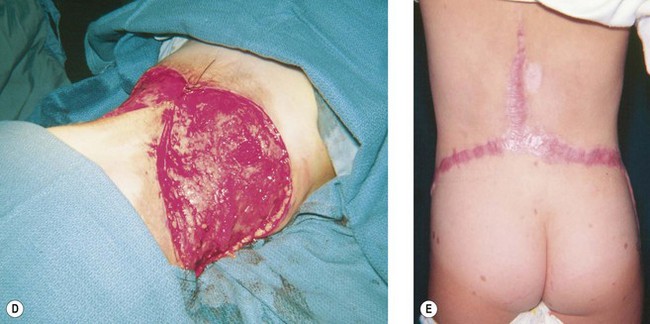
Congenital nevi differ from others in that they already produce pigment at birth (Fig. 31.4A). There is some controversy about whether congenital nevi are precursors of melanoma. Kaplan’s review12 of the literature reported the transformation to melanoma to occur in 2–42% of congenital nevi (Fig. 31.4B). In a retrospective study of 234 melanomas by Rhodes and Melski,13 some of the histologic features of congenital nevi were found among 8% of the melanoma specimens. A systematic review of all studies evaluating the risk of melanoma in congenital nevi was performed by Krengel et al.14 A total of 6571 patients with congenital nevi were followed for at least 3.4 years, and 46 patients (0.7%: range 0.05–10.7%) developed 49 melanomas. Of note, primary melanomas arose inside the nevi in 67% of cases. Using age-adjusted data from the Surveillance, Epidemiology and End Results database, they calculated that patients with congenital nevi carry an approximately 465-fold increased relative risk of developing melanoma during childhood and adolescence. Large congenital nevi (Fig. 31.5), greater than 40 cm in diameter, were associated with the highest risk of developing melanoma, as well as dying from melanoma.14,15 However, the true incidence of the development of melanoma within congenital nevi is difficult to determine as the number of patients in the general population who have congenital nevi but never consult a physician, or eventually undergo excision, is unknown.
On the basis of available information about the potential for malignant transformation, it is a good policy to remove congenital nevi if it can be done without much difficulty (Fig. 31.5). Malignant transformation does not usually occur before adolescence, thus, if the lesion is to be excised, it should be done before adolescence. Because it is difficult to excise nevi from the skin of children under local anesthesia and general anesthesia is often necessary for children younger than 12 years, the risk of complications from general anesthesia should be weighed against the risk of malignant transformation before adolescence. On the other hand, patients may request removal of the lesion to improve their appearance. Despite concerns for appearance, some lesions cannot be completely removed because in doing so we may cause a greater deformity. These lesions may require staged excisions.
Atypical (dysplastic) nevus
The atypical nevus is a clinical diagnosis of a nevus with melanocytes involving the epidermis and dermis that have features suggestive of malignancy. Clinically, it is large (>6 mm), with a macular surface, irregular margin, and variegated color. It may have a background of erythema (Fig. 31.6). These are benign lesions with histologic features that are abnormal. At various times, they have been called atypical nevi or dysplastic nevi. However, a National Institutes of Health Consensus Conference in 1992 recommended the descriptive term “atypical nevus” for the clinical diagnosis and the histologic term “dysplastic nevus” to describe the histologic degree of atypia and architectural disorder.16
To ensure accurate diagnosis, histologic examination of the lesion is required. Microscopically, the dysplastic nevus has melanocytic hyperplasia, with the melanocytes arranged as solitary units or small elongated nests oriented parallel to the long axes of the rete ridges. The melanocytes have nuclear atypia and abundant cytoplasm with a fine “dusty pattern” of melanin deposits.17 Dysplastic nevi are often associated with atypical melanocytic hyperplasia, lymphocytic infiltration, and some evidence of regression. As such, patients with these lesions are believed to be at greater risk for transformation to melanomas.
Atypical (dysplastic) nevus syndrome
Studies at several institutions have found atypical nevi in association with melanoma that has no familial pattern. At the University of Pennsylvania, Elder et al.18 first described this as the dysplastic nevus syndrome in their 1980 report. In the same year, the Yale Melanoma Unit visited the Sydney Melanoma Unit in Australia and documented the presence of atypical nevi in 37% of 296 patients with melanoma who had no known family history.19 Similar atypical nevi were discovered in only 7% of a control population of male prison inmates without any history of melanoma.19 Clinically, these moles were large and resembled the dysplastic nevi of familial melanoma. Biopsies showed a 90% correlation between the histologic diagnosis of dysplastic nevi and the clinical appearance of these atypical moles. The tendency to develop atypical nevi is presumed to have a genetic basis, and the diagnosis of “atypical nevus syndrome” has been applied to patients with a range of phenotypic expressions,20 including just the presence of multiple atypical nevi and no personal or family history of melanoma, to the familial atypical multiple mole and melanoma syndrome.21
B-K mole syndrome
Some prospective studies have shown that melanoma may be associated with a familial distribution in 10–11% of the cases.22 These familial melanomas tend to appear earlier and are distributed among dysplastic nevi over the body, with an excess over the trunk and a deficit over the upper extremities. Clark et al.23 and Reimer et al.24 suggested the role of atypical moles and dysplastic nevi in the development of hereditary melanoma when they described these moles in association with melanomas in seven families. They applied the initials of the first family, which were BK, to name this clinical entity the B-K mole syndrome.
Differential diagnosis
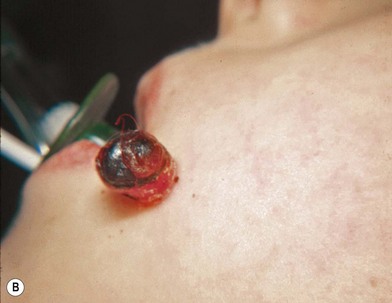
The clinician is faced with the task of differentiating the malignant melanoma from a number of other lesions that may clinically resemble melanoma, such as seborrheic keratosis (Fig. 31.7A), pyogenic granuloma (Fig. 31.7B), and pigmented basal cell carcinoma (Fig. 31.7C). This differentiation may sometimes be more difficult because of a recent growth, bleeding into a lesion, or peripheral inflammation. In these instances, only microscopic examination of the tissue provides the proper diagnosis.
Extensive or radical surgical procedures should not be performed without the proper diagnosis of a melanoma because clinical impressions are not uniformly correct. Epstein et al.25 reviewed 559 patients with black lesions that they believed might be melanomas. They found that their diagnosis of melanoma was correct only a third (38.7%) of the time. Indeed, the most common diagnoses were benign nevi (35%), pigmented basal cell cancer (30%), and benign angiomas or vascular lesions (13%). Only 2% of all the lesions were found to be melanoma. Dermoscopy, the use of a hand-held lens in combination with oil immersion, has been shown to improve diagnostic accuracy in skilled hands.26,27 However, it is used routinely by only 23% of dermatologists.28 In general, the diagnostic work-up of melanocytic lesions will precede the surgeon’s involvement, unless the lesion is in a region of cosmetic concern.
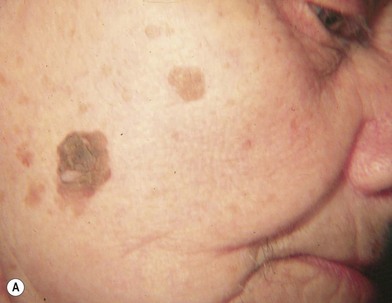
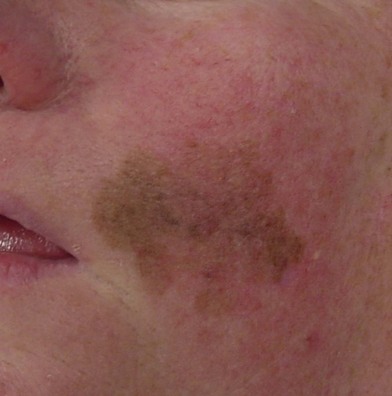
Hutchinson freckle
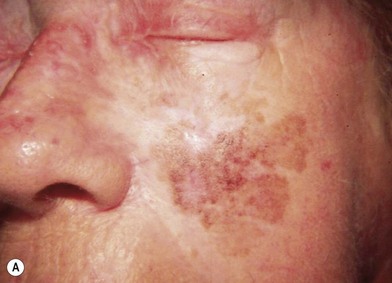
Hutchinson freckle is a flat, brown, macular lesion that may grow at various rates and achieve different shades of pigmentation (Fig. 31.8). This lesion occurs most commonly on the face, neck, and other sun-exposed surfaces of adults in middle age or later. On histologic examination, this lesion appears as an overgrowth of melanocytes at the epidermis–dermis junction. Although lentigo maligna is an in situ melanoma, invasive melanoma may develop within a Hutchinson freckle and is then called lentigo maligna melanoma.
Melanoma
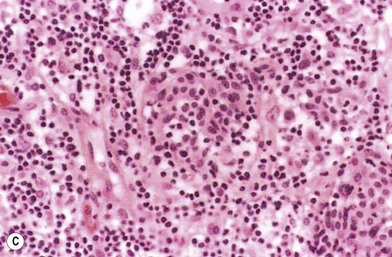
The lesions of melanoma may be flat or nodular, with significant darkening, erythema, or bleeding. On histologic examination, the earliest lesions demonstrate atypical melanocytes migrating above the dermis–epidermis junction and appearing within the upper portions of hair follicles and eccrine ducts. These changes are typical of melanoma in situ.29 Special staining with S100 and HMB45 may be necessary to confirm the diagnosis in cases with histologic features that may be equivocal. However, when even a single atypical melanocyte invades from the dermis–epidermis junction down into the dermis, the diagnosis is melanoma.30
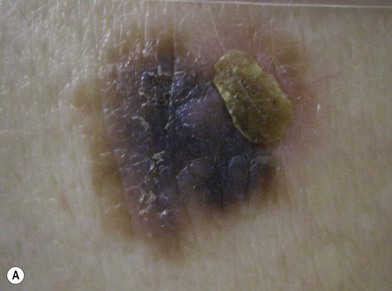
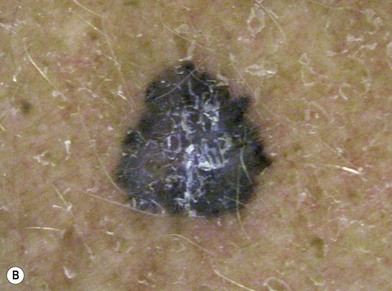
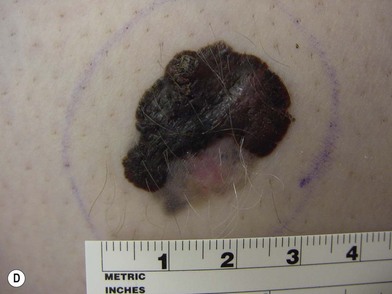
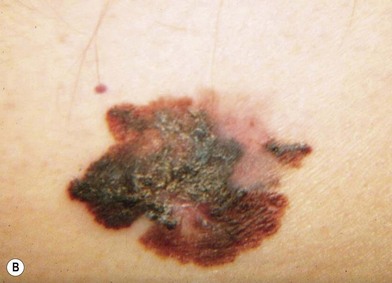
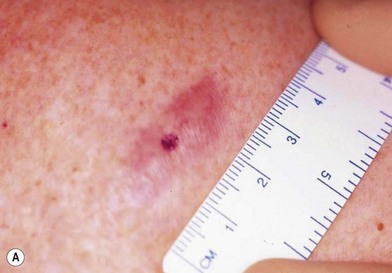
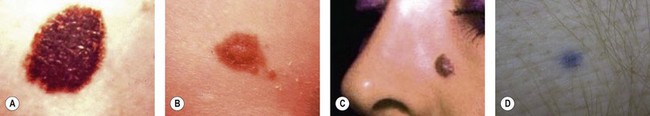
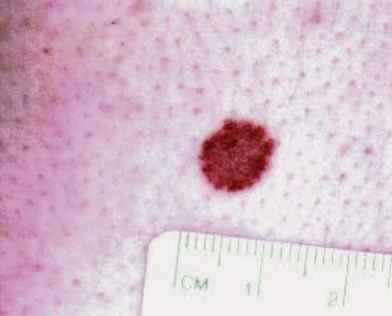
There are clinical features of pigmented lesions that are characteristic for melanoma. These criteria have been promoted by the American Cancer Society as the ABCD Guidelines (Fig. 31.9).
A. asymmetry of the lesion as it grows from a round or oval lesion
B. border irregularity, which is a result of irregular growth rates of different parts of the lesion
C. color changes representing pigment granules deposited at varying depths in the dermis, depending on the rate of invasion
In addition to the above ABCD criteria, a review of the literature recommended the addition of E for “evolution,” in order to emphasize the significance of evolving pigmented lesions in the natural history of melanoma, especially given the existence of small-diameter (≤6 mm) melanomas.31 In treating patients with suspicious pigmented lesions, physicians should be attentive to changes (evolving) of size, shape, symptoms (itching, tenderness), surface (especially bleeding), and shades of color. An investigation of the recognition process of melanoma by 135 dermatologists revealed that most dermatologists rely more on the lesion’s overall pattern, the “ugly duckling sign” (i.e., unique appearance relative to the patient’s other nevi), and recent change according to the patient, rather than the better-known ABCD algorithm.32 This observation lends support to the addition of an E category for evolution of melanocytic lesions by the nondermatologist.
Based on existing literature, most patients who present to their dermatologist are unaware of an existing melanoma. Most melanomas (56.3%) detected in the general dermatology practice are found by the dermatologist during routine physical examination, and are not part of the presenting complaint.33 Numerous studies34–36 demonstrate that physicians are more likely to detect melanomas at a thinner stage than nonphysicians. In these studies, there was a significant difference between the thickness of physician-detected melanomas (0.23–0.68 mm) and those detected by patients or their spouses (0.9–1.43 mm).
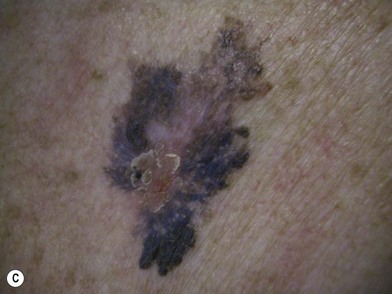
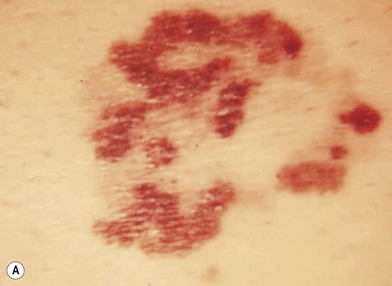
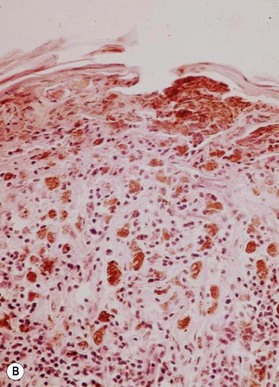
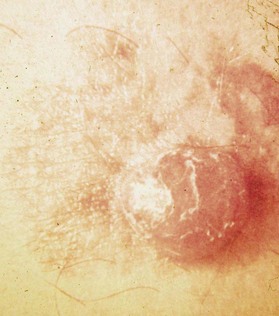
An occasional, and potentially reassuring, feature of melanoma is intralesional depigmentation (Fig. 31.10). This is a manifestation of immunologic regression of the tumor as a result of the destruction of the melanoma cells by the host’s immune response. The histologic examination of only a section through the depigmented portion may be misread as an inflammatory reaction. However, deposits of residual melanin granules may exist in the depigmented portion (Fig. 31.10B), and histologic examination of sections through the adjacent pigmented portion may reveal the true diagnosis of the melanoma.
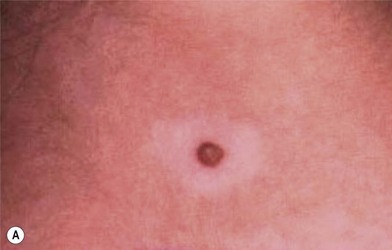
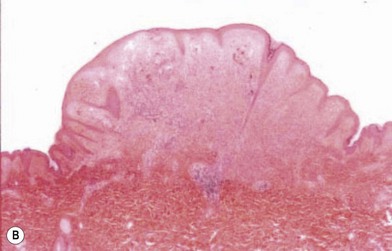
Nevertheless, depigmentation does not always indicate melanoma: a halo nevus (Fig. 31.11A) is a benign lesion with a peripheral ring of depigmentation.37 Histologic examination of the halo portion shows lymphocytic infiltration without pigment granules (Fig. 31.11B). Further evaluation of the lesion and surrounding tissue shows no evidence of cells of malignant melanoma (Fig. 31.11C).
Multiple primary melanomas
Multiple primary melanomas have been reported to occur among 3% of melanoma patients.38 The risk for a second melanoma in a patient with one melanoma approaches 4–5%.39 However, with a positive family history of melanoma, the risk for multiple primary melanomas rises to 10% or more.40 The highest risk of all appears to be in individuals who have a family history of melanoma in one or two first-degree relatives and who have clinical evidence of dysplastic nevi, suggesting a probability approaching 100% in due time.41 Although multiple primary melanomas may be found among 10% of the patients, Ariyan et al.42 reported that half of these subsequent melanomas are in situ, and the vast majority of the rest are less than 1.0 mm thick; thus, they did not seem to affect cure rates.
Classification/staging of disease
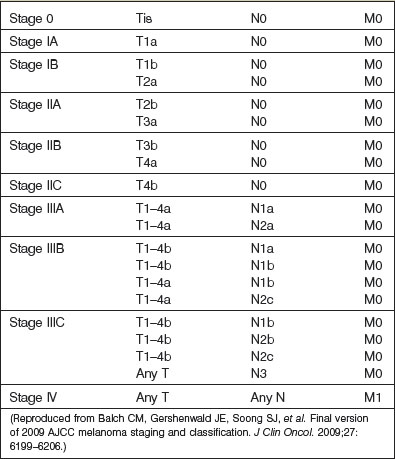
The current (2010), seventh edition of the American Joint Committee on Cancer (AJCC) staging system relies upon data related to the primary tumor (T), regional lymph nodes (N), and metastases (M). Also known as the TNM system, this staging system was developed based upon analysis of over 38 900 patients with cutaneous malignant melanoma43,44 (Tables 31.2, 31.3).
Table 31.2 Cutaneous melanoma TNM staging
| T classification | Thickness | Ulceration status |
| Tis | Not applicable | Not applicable |
| T1 | ≤1.0 mm | a: without ulceration and mitosis <1/mm2 |
| b: with ulceration or mitosis ≥1/mm2 | ||
| T2 | 1.01–2.0 mm | a: without ulceration |
| b: with ulceration | ||
| T3 | 2.01–4.0 mm | a: without ulceration |
| b: with ulceration | ||
| T4 | >4.0 mm | a: without ulceration |
| b: with ulceration | ||
| N classification | No. of metastatic nodes | Nodal metastatic mass |
| N1 | 1 node | a: micrometastasis* |
| b: macrometastasis† | ||
| N2 | 2–3 nodes | a: micrometastasis* |
| b: macrometastasis† | ||
| c: in transit met(s)/satellite(s) without metastatic nodes | ||
| N3 | 4 or more metastatic nodes, or matted nodes, or in-transit met(s)/ satellite(s) with metastatic node(s) | |
| M classification | Site | Serum lactate dehydrogenase |
| M1a | Distant skin, subcutaneous, or nodal metastases | Normal |
| M1b | Lung metastases | Normal |
| M1c | All other visceral metastases | Normal |
| Any distant metastasis | Elevated |
* Micrometastases are diagnosed after sentinel or elective lymphadenectomy.
† Macrometastases are defined as clinically detectable nodal metastases confirmed by therapeutic lymphadenectomy or when nodal metastasis exhibits gross extracapsular extension.
(Reproduced from Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206.)
Histologic subtypes of melanoma
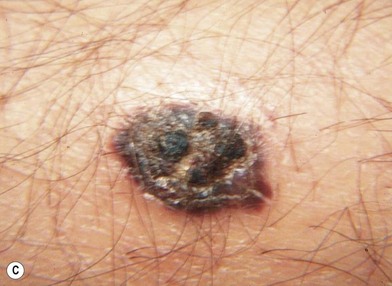
While morphology or histologic subtype does not necessarily correlate with clinical behavior, subclassification is important for pathologic recognition and diagnosis. Melanoma may be classified morphologically into four major growth patterns: lentigo maligna, superficial spreading, nodular, and acral lentiginous types (Fig. 31.12). Superficial spreading melanoma (Fig. 31.12B) represents 50–80% of all the types and is characterized by growth in the radial (horizontal) phase for a period of years before evolution into the vertical growth phase. Nodular melanoma (Fig. 31.12C), on the other hand, evolves into the vertical growth phase early in its development and represents 20–30% of the group but in some series may compose the majority of the lesions.45 Lentigo maligna melanoma (Fig. 31.12A) is differentiated from superficial spreading melanoma and nodular melanoma by its location on sun-exposed surfaces of the body and within pre-existing lentigo maligna (Hutchinson freckle). This morphologic type of melanoma was believed to have a better prognosis than other types by virtue of a different biologic behavior, but it has been shown to have a prognosis identical with that for superficial spreading melanoma with comparable depths of invasion.46 It has been shown that lentigo maligna melanoma merely grows in a horizontal fashion more than in a vertical fashion, resulting in thinner lesions than superficial spreading melanoma or nodular melanoma, which is the reason for its purported better prognosis.
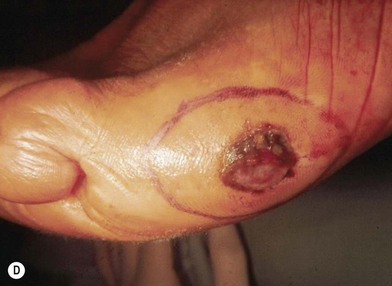
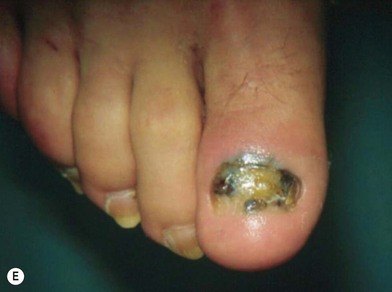
Acral lentiginous melanoma appears on the palms of the hands, soles of the feet (Fig. 31.12D), subungual areas of the fingers and toes (Fig. 31.12E), and webspaces.47 The importance of subungual melanoma is that it is often erroneously believed to be a fungal infection, and appropriate treatment may be inadvertently delayed because of a delay in obtaining diagnostic biopsy. Presumably due to delayed diagnosis, this type of melanoma has the lowest 5-year survival rates of all histologic variants, generally found to be in the range of 10–20%.22,48
While older studies suggested that prognosis may be associated with histologic subtype (e.g., patients with nodular melanomas were thought to have a worse prognosis than patients with superficial spreading melanomas),49 more recent multivariate analyses demonstrate that these prognostic differences are more likely due to other histologic features (i.e., tumor thickness and ulceration).50
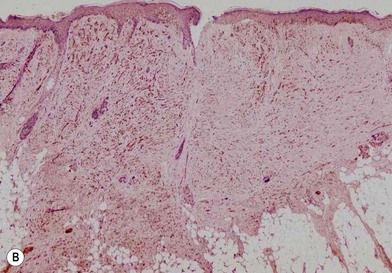
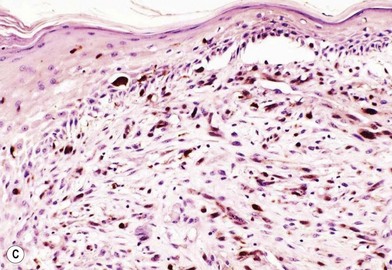
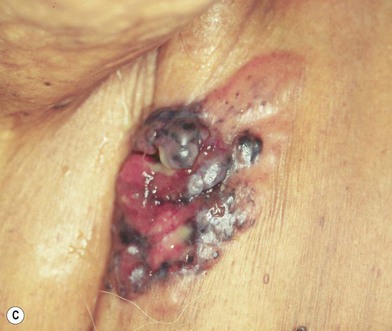
Another less common clinical variant of melanoma, desmoplastic melanoma, usually does not produce pigment, and grows on the external surfaces of the skin. It may have the appearance of a hypertrophic scar (Fig. 31.13A) at a location where the patient does not recall having had an injury to the skin. It must be differentiated clinically from a dermatofibroma and other benign or malignant tumors of the dermis. Histologic examination reveals a cicatricial growth of the lesion with spindle cell variants of malignant melanocytes (Fig. 31.13B).51–53 This histologic subtype must be differentiated from amelanotic melanoma (Fig. 31.14), which is simply a variant of nodular or superficial spreading melanoma that is not producing sufficient pigment granules to appear as a pigmented lesion. In one series of melanomas, the incidence of amelanotic melanoma was found to be 1.8%.54
Histopathologic factors of prognostic significance
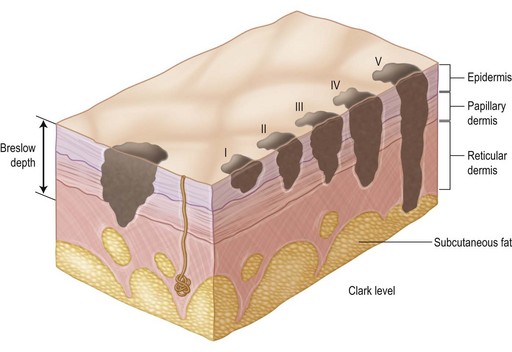
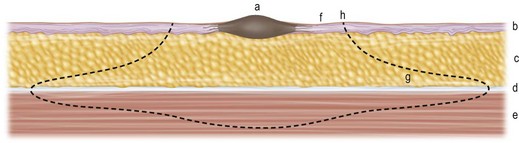
With respect to analysis of the primary lesion alone, the depth of invasion of melanoma into the dermis has been shown to be the most powerful determinant of outcome. In 1965, Mehnert and Heard55 reported the earliest correlation of depth with prognosis. A few years later, Clark et al.56 described the following system of levels for the classification of depth of invasion into the dermis (Fig. 31.15):
Level I: in situ melanoma; limited to the dermis–epidermis junction
Level II: invading the papillary dermis but without expansion of this layer
Level III: invading and expanding the papillary dermis but not into the reticular dermis (to the interface of the papillary–reticular dermis)
Level IV: invading the reticular dermis, but not into the subcutaneous fat
Level V: invading the subcutaneous fat or the associated subreticular tissues.
The difficulty with this classification system is the qualitative and somewhat subjective nature of determining the depth of invasion. Various pathologists examining a histologic slide of mid-dermal invasion often disagree on the Clark level of invasion; some may call it a level III, while others call it a deep level II, and still others call it an early level IV invasion. As a result of this difficulty, Breslow57 reported a method of quantitative measurement that employs a simple and readily reproducible system of microstaging. According to Breslow, the melanoma’s depth of invasion is measured in tenths of millimeters as a thickness from the surface of the tumor in the epidermis to the deepest tumor cell identified by means of an ocular micrometer on the microscope. In a number of studies using multivariate analyses,43,45 Breslow’s method has been shown to be the most powerful prognostic indicator for survival in early-stage melanoma (i.e., disease limited to primary tumor). Additional factors shown, in appropriate multivariate analyses of several thousand patients, to be associated with recurrence and survival include: ulceration in the lesion, the mitotic rate within the lesion, the patient’s age and sex, the site of the primary lesion, and the morphologic type of melanoma.43,58
T category of TNM staging system
The histopathologic factors incorporated into the T category of the 2010 TNM staging system are the thickness of the primary tumor, i.e., the Breslow depth,57 the presence or absence of ulceration of the overlying epithelium, and the mitotic rate.43,44
The thickness of the primary tumor (T) defines four categories (Table 31.2):
Increasing tumor thickness is closely correlated with poorer prognosis. The 10-year survival decreases progressively, from 96% for patients with primary lesions <0.5 mm thick to 54% for patients with lesions 4.01–6.0 mm thick.43,44
The T categories are further subdivided into “a” or “b” based upon the presence or absence of ulceration and the mitotic rate (Table 31.2). Ulceration is defined as the absence of an intact epithelium over the melanoma. Outcomes in patients with ulcerated primary tumors are worse than in patients with primary melanomas of the same thickness but without ulceration. The mitotic rate was incorporated into the 2010 TNM staging system based upon the observation that it was the second most important prognostic factor in over 11 000 patients with localized melanoma analyzed in the AJCC Melanoma Staging Database. In that series, there was a highly significant correlation between increasing mitotic rate and declining survival rates (P < 0.0001). For instance, for melanomas less than 1.0 mm thick, the 10-year survival rate was 93% for those with <1 mitosis/mm2 and 48% for those with >20 mitoses/mm2.43,44 The recommended approach to determining the mitotic rate is to identify the area of dermis containing the most mitoses (the “hot spot”), and average the number of mitoses within a 1-mm2 area of the hot spot.44 The substaging according to mitotic rates applies only to T1 lesions.
With respect to prognostic classification (Table 31.3), stage I melanoma defines patients with low-risk melanomas (T1a–T2a) without evidence of regional or distant metastases. Further subdivision (stages IA and IB) is based upon characteristics of the primary tumor. Stage II melanoma includes primary tumors at a higher risk of recurrence or metastasis (T2b–T4b) without evidence of surgical or distant metastases. Similarly, stage II subdivision (IIA, IIB, IIC) is based upon characteristics of the primary tumor.43,44 Details of more advanced stages of melanoma will be covered in the next section.
In-transit and regional lymph node disease
Local metastatic spread of cutaneous melanoma is known to occur through lymphatic channels in the majority – approximately 90% of cases.59 Direct hematogenous dissemination of melanoma, more difficult to assess and treat, is another less common route of metastasis. There are a number of clinical and pathologic characteristics of regional spread of metastatic melanoma that have become incorporated into the prognostic staging system for primary melanoma, namely satellite/in-transit metastases and sentinel lymph node status.60
Intralymphatic spread of melanoma in the skin can present/manifest as satellite lesions, also known as microscopic satellitosis, or in-transit metastases, which are skin or subcutaneous metastases more than 2 cm from the primary lesion.50 Microscopic satellitosis is seen in up to one-third of primary melanoma lesions greater than 3 mm thick, compared to less than 5% of thinner lesions.60 The presence of microscopic satellitosis and in-transit metastases has been shown to carry a poorer prognosis, similar to the presence of regional lymph node metastases.43,44 Thus, in the current AJCC system, patients with microscopic satellitosis or in-transit metastases are upstaged to the level of a patient with established lymph node metastases (Tables 31.2 and 31.3).
Spread of melanoma to the regional lymph nodes portends a poorer prognosis. As melanoma is known mostly to metastasize through lymphatic channels (and the first sites of metastasis are typically regional lymph node basins), evaluation of the regional lymph node basins has become critical in the staging of melanoma. Unfortunately, clinical evaluation of the regional lymph nodes is often inaccurate: as many as 20% of clinically node-negative patients have metastatic involvement on pathologic evaluation, and up to 20% of those with clinically positive nodes are pathologically negative.61
Mostly of historical significance, elective lymph node dissection (ELND) has largely been supplanted by sentinel lymph node biopsy (SNLB). However, brief mention is warranted. In the setting of clinically node-negative disease, ELND involves removal of all lymph nodes in a suspected draining basin. The rationale is that removal of subclinical regional disease may provide a survival benefit over a therapeutic node resection performed after regional disease becomes clinically evident. Performance of this procedure is now controversial, in the era of lymphoscintigraphy and SNLB, especially as most melanomas are being diagnosed early (thinner and less invasive), and the morbidity associated with a completion lymphadenectomy can be quite significant. Additionally, while a few retrospective analyses suggested ELND might improve survival by 25–40%,62 most prospective randomized trials have not demonstrated a survival benefit from ELND.63
Lymphoscintigraphy (lymphatic mapping) and sentinel lymph node biopsy
In the skin, as in all parts of the body, arterial blood pressure diffuses serum and nutrient material out of the vessels into the interstitial tissue to nourish the cells. The breakdown products of metabolism are then picked up by the veins and taken back into the systemic system. Because the pressure in the arteries is greater than in the veins, more of this vascular fluid is diffused into the tissue than is taken away by the vein. To avoid the consequences of edema, lymphatic vessels (the micro sump pumps of the system) draw away this excess fluid and take it to the regional lymph nodes to filter the product before returning it to the systemic vascular system. This filtering function of the lymph nodes allows detection and attack of foreign bacteria, antigens, and cancer cells. It was this principle that permitted Sappey64 to show the lymphatic patterns of the human body in 1874 by injecting mercury into the skin. Sappey’s lines are a helpful guide to determine the likely directions of lymphatic spread. Subsequent experience has shown that lesions located more than 2 cm above or below a “belt line” drawn through the umbilicus usually drain to the axillary or groin nodes, respectively. Lesions more than 2 cm on either side of the midline drain to the lymph nodes on that respective side. Lesions within 4 cm of the vertical and horizontal bands may go to any one of the pairs of options (Fig. 31.16).
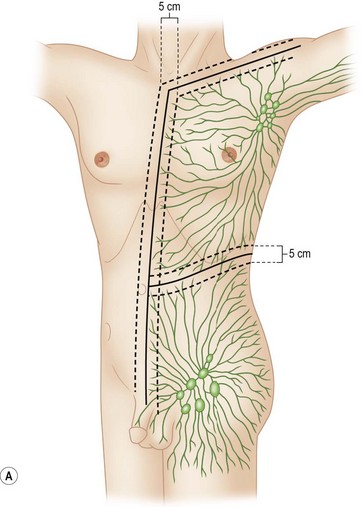
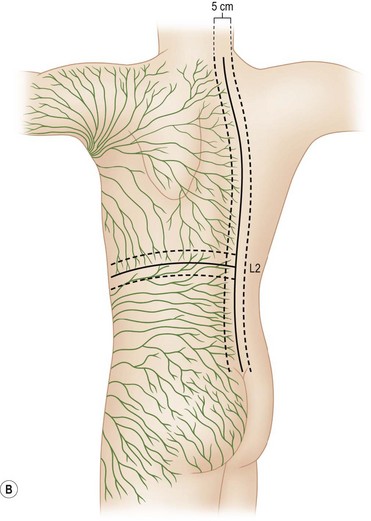
Fig. 31.16 (A, B) Lymphatic drainage as predicted by Sappey’s lines.
(Redrawn from Sugarbaker EV, McBride CM. Melanoma of the trunk: the results of surgical excision and anatomic guidelines for predicting nodal metastasis. Surgery. 1976;80:22.)
As a result of potentially unclear lymphatic drainage patterns, Sherman and Ter-Pogossian65 introduced lymphoscintigraphy in 1953. They injected radiocolloid gold (198Au) intradermally and used a gamma counter to detect the concentrated colloidal isotope in the filtering lymph nodes. This technique has since been modified with various other isotopes and colloids of various particle sizes for specific diagnostic purposes. The intent of each of these modifications is to identify the lymphatic vascular pattern in the tissue being evaluated.
The technique of lymphatic mapping is helpful for predicting the pattern of spread for cutaneous melanoma metastases to regional draining lymph node basins, and it may be helpful in detecting metastases in melanoma of the extremities.66 In particular, the test may be useful in patients with T2 or thicker lesions of the lower extremity for evaluation of the iliac and pelvic lymph nodes to determine the extent of lymphadenectomy that may be indicated. The iliac nodes should certainly be removed if they appear to be involved, but a pelvic lymphadenectomy is not indicated if the para-aortic lymph nodes are involved because cure is unlikely in these cases.
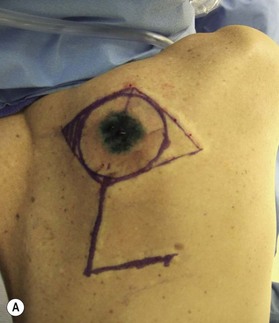
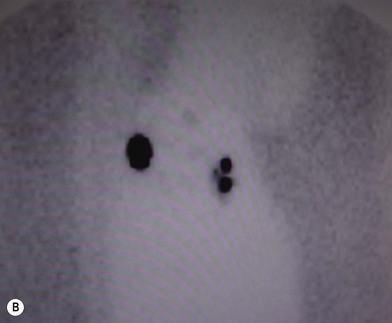
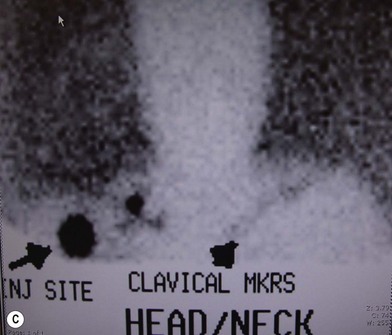
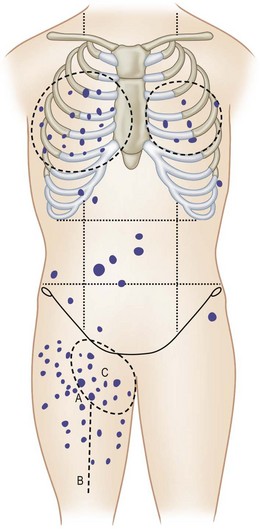
The sites of lymphatic spread from melanoma at other locations, including the trunk and head and neck regions, may be also evaluated by radionuclide lymphoscintigraphy.67 Several radiocolloids have been employed for lymphoscintigraphy, including gold, sulfur, and antimony. Antimony sulfide colloid and technetium sulfur colloid have been found to be safe, and both give reliable information for determining appropriate lymph nodes for elective dissection among patients with truncal or head and neck melanomas (Fig. 31.17).
A lymphoscintigram can demonstrate predicted as well as unexpected patterns of lymph node drainage from lesions at primary sites. The test has been demonstrated as a reliable predictor of the sites of nodal involvement. In a prospective study of 51 consecutive patients with primary melanomas greater than 1 mm thick and observed for a mean of 45 months, 23% of the 35 patients who chose to undergo elective lymphadenectomy were found to have micrometastases to these lymph nodes; all were in the node groups detected by the lymphoscintigram.68 During the several years of their follow-up, 5 of the 16 patients (31%) who chose to be observed eventually developed clinical evidence of nodal metastases; in each case, the nodes that were involved with tumor were at the very sites of drainage predicted by the lymphoscintigrams that were performed at the time of initial diagnosis (Table 31.4). During the 7-year interval of follow-up, no patient in either group developed metastases to any nodes not predicted by lymphoscintigraphy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 inch (>6 mm).
inch (>6 mm).