Management of Portal Vein Thrombosis
Ashish Singhal
Flavio Paterno
Shimul A. Shah
DEFINITION
Portal vein thrombosis (PVT) refers to the thrombotic occlusion of the portal lumen, which can affect the intrahepatic and the extrahepatic venous tracts (FIG 1).
Although many classifications of PVT exist, its importance lies in the surgical approach employed, which is determined by the extent of thrombosis, and the presence/adequacy of collateral vessels that can potentially be used for extraanatomic reconstructions to establish portal venous flow.
INCIDENCE
The incidence of PVT in patients with end-stage liver disease (ESLD) varies from 5% to 15% with up to half of all patients diagnosed at surgery. The majority of patients have partial thrombosis.
The incidence of thrombosis occurring while on the liver transplant waiting list is 7% for a mean waiting time of 12 months.
RISK FACTORS
In noncirrhotic patients, PVT is most often related to prothrombotic states (myeloproliferative neoplasms and/or inherited coagulation disorders).
In cirrhotic patients, portal hemodynamics is the main factor leading to thrombosis. The characteristic parenchymal changes of cirrhosis as well as changes in vasoreactivity result in increased intrahepatic vascular resistance and reduced portal flow. Both pro- and anticoagulant factors are decreased in cirrhosis, resulting in a fragile compensated hemostatic balance that may easily tip toward a hypo- or hypercoagulable situation, causing either hemorrhagic or thrombotic complications.
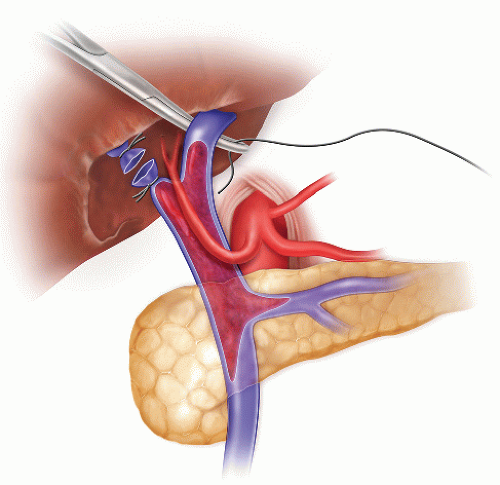
FIG 1 • PVT identified at the time of dissection and ligation of the portal vein. High ligation of the portal vein allows a better control of the vein during the thrombectomy.
Low platelet count seems to be an independent predisposing factor for PVT in cirrhosis, although it could be a proxy for the degree of portal vascular resistance (portal hypertension).
Cirrhotic patients with PVT, compared to those without PVT, have an increased prevalence of factor V Leiden, methylene tetrahydrofolate reductase C677T polymorphism, and prothrombin gene mutations, the latter being particularly frequent. However, only the mutation 20210 of the prothrombin gene is independently associated with PVT.
PVT is more common in male; patients with autoimmune, chronic active hepatitis; cryptogenic cirrhosis; alcoholic liver disease; and patients with previous treatment of portal hypertension-related bleeding (sclerotherapy, surgery, and splenectomy). The presence of at least one risk factor, including male sex, previous treatment of portal hypertension, Child C class, and the presence of alcoholic liver disease, increases the rate of PVT from 6.6% (absence of risk factors) to 12.5%.
Presence of hepatocellular carcinoma (HCC) constitutes a risk factor for PVT. Tumor invasion involving the branches and/or the trunk of the portal vein is a possible source of portal vein obstruction in patients with HCC. Macroscopic vascular invasion by the tumor is a definitive contraindication for transplantation. Therefore, in candidates for transplantation with HCC and portal vein obstruction, a clear distinction between tumor invasion and thrombosis should be made.
IMAGING AND OTHER DIAGNOSTIC STUDIES
Doppler ultrasonography (US) and multiphasic (arterial and portal) helical computed tomography (CT) are the investigation of choice.
Doppler US is highly accurate at detecting thrombosis involving the trunk of the portal vein and in intrahepatic branches. It provides additional information concerning portal flow and its direction. Doppler US may be recommended every 3 months during waiting time, whenever possible.
CT clearly displays the superior mesenteric vein (SMV), spontaneous portosystemic shunts, renal veins, and the inferior vena cava (IVC).
Magnetic resonance imaging (MRI) is an alternative to CT in patients with impaired renal function or depending on local expertise and preference. However, the definition is lower than that of CT, especially in patients with tense ascites.
SURGICAL MANAGEMENT
The main objective of treatment is to achieve at least partial recanalization so that portal flow to the graft can be restored through conventional end-to-end portal vein anastomosis. Also, to prevent further extension of the thrombus during waiting time, especially to the SMV.
Anticoagulation
Anticoagulation in cirrhosis is justified by the preserved balance between pro- and anticoagulant factors, even when coagulation factors are decreased. With partial thrombosis, complete recanalization may be achieved in 40% to 75% of patients, whereas less than 10% experience an extension of the thrombus. The rate of recanalization is significantly higher in patients receiving anticoagulation than in control patients who do not. However, no good data is available regarding bleeding risks in cirrhotic patients who could be at risk of excessive anticoagulation. Anticoagulation is not considered effective for chronic PVT in cirrhotic patients.
Transjugular Intrahepatic Portosystemic Shunt
The objective of transjugular intrahepatic portosystemic shunt (TIPS) is to recanalize the portal vein and, subsequently, prevent rethrombosis by restoring portal flow through a low-resistance shunt. It clearly appears that the feasibility of TIPS varies according to the extent of thrombosis. Technical failure may be related to the absence of visibility of intrahepatic branches of the portal vein, transformation of the portal vein into a fibrous cord, and extension of the thrombus to the SMV. TIPS may be feasible in some patients with cavernoma. Ideally, TIPS insertion and recanalization might be associated with disrupture of the thrombus and mechanical thrombectomy.
TECHNIQUES
THROMBENDOVENECTOMY
The portal vein thrombus is usually organized with a significant fibrotic component strongly attached to the intima. An effective approach in these cases is a thrombendovenectomy. This technique is similar to a thrombo-endarterectomy: The intima is removed together with the clot.1
The PVT is usually addressed at the end of the hilar dissection. The hepatic artery and the bile duct are tied and divided. The portal vein is dissected circumferentially from the pancreas up to its right and left branches.
It is critical to mobilize a long segment of portal vein in order to gain adequate control of the vein during the thrombectomy (FIG 2). In case of extensive PVT, the retropancreatic segment of the portal vein should be exposed.
Before starting the thrombectomy, it is important to coordinate with the anesthesia team and have packed red blood cells available in case of large bleeding.
The right and left branches of the portal vein are ligated and transected while the main trunk of the portal vein is occluded manually. Three tonsil clamps are applied on the margins of the portal vein and hold the vein wall in tension. The clot is carefully separated from the portal vein wall with an intimal elevator or a tonsil clamp (FIG 3). Once the upper part of the clot is freed circumferentially, it is grabbed with a ring clamp or a tonsil clamp (FIG 4). The thrombus is dislodged by rotating the clamp first clockwise and then counterclockwise and by pushing the clot inward, toward the origin of the portal vein. After most of the clot is mobilized, it can be pulled out (FIG 5).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








