2 Management of lower extremity trauma
Synopsis
 Lower extremity trauma is common, and often associated with other injuries.
Lower extremity trauma is common, and often associated with other injuries.
 An Advanced Trauma Life Support (ATLS) approach is required in order to evaluate a patient with lower extremity injuries properly.
An Advanced Trauma Life Support (ATLS) approach is required in order to evaluate a patient with lower extremity injuries properly.
 Initial management includes fracture reduction to reduce bleeding, and appropriate radiologic investigations.
Initial management includes fracture reduction to reduce bleeding, and appropriate radiologic investigations.
 External fixation is useful in the temporization of complex fractures until definitive fixation can be achieved.
External fixation is useful in the temporization of complex fractures until definitive fixation can be achieved.
 Evidence-based practice with regard to antibiotics, anticoagulation, and timing of reconstruction is recommended.
Evidence-based practice with regard to antibiotics, anticoagulation, and timing of reconstruction is recommended.
 A multidisciplinary approach is required in the acute and rehabilitative phases of a patient with lower extremity trauma.
A multidisciplinary approach is required in the acute and rehabilitative phases of a patient with lower extremity trauma.
Introduction
Major injuries are a significant cause of death and disability worldwide. Injuries are among the leading causes of death in the US, with unintentional injuries as the fifth leading cause of death in all ages.1 During the years 1999–2007 unintentional injuries were the leading cause of death for those aged 1–44 years, and the third leading cause of death in those aged 45–54 years.2 In the most recent National Vital Statistics Report (2007), motor vehicle collisions (MVCs) were responsible for 23% of injury-related deaths.3 The World Health Organization ranked MVCs as the ninth leading cause of disability-adjusted life years, and predicted an increase in ranking to third by 2030.4 According to the National Trauma Bank’s Annual Report (2009),5 the greatest number of incidents occur between the ages of 25 and 34 years, with a case-fatality rate of 3.70. The highest case-fatality rate occurred in those >85 years of age (7.72). Males have a higher case fatality rate beyond age 15.
The management of a patient with lower extremity trauma involves a multidisciplinary approach. Following the initial provision of emergent trauma care and evaluation by orthopedic and general surgery/vascular surgery colleagues, the plastic surgeon is often involved in the management of extremity coverage and reconstruction of both salvaged and amputated limbs. This is often done in conjunction with orthopedics, thus emphasizing Levin’s concept of the “orthoplastic approach.”6 The current literature is constantly evolving to reflect changes in practice regarding the criteria for limb salvage, timing of reconstruction, and appropriate supportive and adjunctive patient care.
Historical perspective
In the ancient era, compound fractures were considered a fatal injury and not treated.7 This was prior to the advent of amputation. It was not until the time of Hippocrates (460–370 bc) that amputation was first mentioned; Hippocrates suggested the use of amputation as a “last resort” in the treatment of ischemic gangrene.8 In this circumstance, he recommended that one could “complete” the amputation already underway by cutting through the necrotic region to minimize the risk of hemorrhage and patient discomfort.8
Aurelius Conrelius Celsus (25 bc–50 ad) was the first to address important principles in wound care, including cleaning wounds of extraneous material and properly placing sutures to relieve tension.7 He recommended amputation through viable tissue, and proper shaping of the stump, as well as the use of the “hemostatis ligature.” In the early first century ad, hemostasis was achieved through ligation as well as through cautery using hot irons or boiling oil.
The advent of war brought with it significant advances in the management of wounds and traumatic injuries. Warfare incorporated the use of gunpowder in 1338, and this changed the nature and severity of wounds requiring medical attention.7 Gunpowder was thought to be poisonous, and many attempts to extract it were made, including the use of setons, oil-moistened lint plugs, and the manual extraction of shot or bone splinters.9
It was perhaps the work of Ambroise Paré (1509–1590) that modernized trauma surgery and wound care. His initial enthusiasm for cautery was abandoned when his remedy “olye of Elders scalding hot” became unavailable and he was forced to use an alternative dressing comprised of egg yolk, rose oil, and turpentine, with better results.10 He reserved amputation for gangrenous extremities, but when required, he paid careful attention to the ligation of large blood vessels (following a brief period of permissive bleeding to reduce inflammation), cutting the bone shorter to allow good muscular coverage of the stump, and the use of large sutures.10 He was the first to take care to create a stump amenable to prosthetic fitting (e.g., cutting the tibia five finger-breadths below the knee). He was also involved in the production of several extremity prostheses, as well as the design of a ligation clamp named the crow’s beak (bec de corbin).
It was during the 17th and 18th centuries that the concept of primary and secondary amputation of a traumatic limb came to fruition. This coincided with published work by John Hunter that demonstrated a better understanding of physiology and disease processes such as shock, phlebitis, and pyemia.11 Hunter documented conditions for primary amputation that included massive crush injury, partial amputation, and uncontrolled bleeding. The relevance of wound necrosis and the importance of debridement were established by Pierre-Joseph Desault in the late 18th century.7 One of his trainees, Dominique-Jean Larrey (1766–1842) went on to become Napoleon’s chief surgeon, and further contributed to the development of trauma surgery. It was Larrey who pushed for early (primary) amputation as he observed that the first 24 hours were the only “hours of calm that nature would allow.”12 With this in mind, it was Larrey who formed the “flying ambulance” corps of the French army, promoting the prompt evacuation of wounded soldiers.
Over the next few centuries, several medical advances improved the care of the traumatized lower extremity. In particular, the advent of anesthesia (ether (1846) and chloroform (1847)) allowed the surgeon to focus more on technique rather than on speed. This also allowed for fewer surgical assistants, who had previously been required to hold the patient and stabilize the limb during surgery. Secondly, with the advent of antisepsis and microbiology, surgeons became aware of the concept of infection. Joseph Lister (1827–1912) proposed the treatment of compound fractures using occlusive dressings soaked in carbolic acid.13,14 Initially, many surgeons failed to adopt Lister’s principles in their approach. One exception was the Germans, who later went on to develop stream sterilization, face masks, and sterile operating gowns.7
With the onset of the 20th century, warfare gradually became modernized as experience was gained in the Civil War, followed by the First and Second World Wars. With advances in ballistics, once again the nature of injury evolved and produced mutilation that had not previously been encountered. The medical field responded with advances in bacteriology (i.e., the discovery of sulfa antibiotics, and later on, penicillin), radiology, and the establishment of acute surgical management and resuscitation protocols that included early debridement and volume repletion with saline and blood. The role of amputation was also further debated, with the abandonment of prophylactic limb amputation in cases of compound fractures.7 It was through war that the specialty of plastic surgery gained greater acceptance, as there was a need for the reconstruction of combat injuries.15 Prior to the world wars, reconstruction was primarily aimed at the reconstruction of facial defects. Work by surgical pioneers Sir Harold Gillies and Vilray Blair during the First World War popularized the use of tube flaps and delayed flap transfer.15 Similarly, advances in orthopedic surgery made by Sir Robert Jones and Hugh Thomas popularized their now commonly used splints in the stabilization of lower extremity fractures. In addition, Jones was instrumental in the training of over 400 orthopedic surgeons, and the establishment of rehabilitation medicine.7
Basic science and disease process
The body’s response to trauma is a complex orchestration of inflammatory and immune responses. There is an interplay between those mediators produced at the site of injury (e.g., cytokines, growth factors, nitric oxide, and platelet-activating factors) and the activation of local and systemic polymorphonuclear neutrophils, lymphocytes, and macrophages.16 The changes in hemodynamics, immune and metabolic responses following trauma are largely due to the effects of cytokines. Cytokines act through specific cellular receptors, and activate intracellular signaling pathways that regulate gene transcription.
The amplitude of the inflammatory response is often related to the severity of the injury. In addition to the initial response to the trauma, a secondary response, or secondary hit phenomenon, occurs with further interventions, including surgical management of fractures and reconstruction.17–19 There has been increasing focus on methods of avoiding or decreasing the effect of this second hit.
Diagnosis and patient presentation
Lower extremity trauma is often seen in the setting of polytrauma, emphasizing the need to follow protocols during the assessment and management of these patients. Vigilance to rule out other injury is important, as complex lower-extremity injuries can be a significant form of distracting injury. Death due to trauma is often described as having a trimodal distribution.20 Immediate death is attributed to central nervous system etiologies, including severe brain injury and high spinal cord injury, or major vessel/cardiac injury. Salvage of these patients is rare, emphasizing the need for prevention of such injuries. The second peak occurs within minutes to hours, and deaths are comprised of subdural/epidural hematomas, hemopneumothorax, intra-abdominal major-organ lacerations, pelvic fracture, and hemorrhage from multiple combined injuries. Finally, the third peak occurs within days to weeks, and is due to sepsis or multiple-organ failure, pulmonary embolism, and unrecoverable head injury.
The assessment of the patient with lower extremity trauma starts with triage, to insure that the patient is treated in the appropriate facility capable of managing the injuries. The ultimate goal is to provide timely care (in the “golden hour”) to minimize secondary injury. In patients presenting with lower-extremity injuries associated life-threatening injuries may be present in 10–17%.21 It is therefore paramount to use a protocol-based assessment of the patient such as the ATLS algorithm. Beginning with the primary survey, the airway is maintained or secured while providing cervical spine control, followed by assessment of breathing. Once this is complete, a circulatory exam is carried out, ensuring proper end-organ perfusion and peripheral circulation, as well as control of bleeding. The appropriate monitors are placed, two large-bore intravenous lines are inserted for fluid resuscitation, and a Foley catheter is inserted once a proper rectal/genitourinary screening exam is complete. Any areas of obvious hemorrhage are compressed, and splints or pelvic binders are placed to stabilize orthopedic injuries. Following this, a screening neurological exam is performed to assess the patient’s level of consciousness (Table 2.1), cranial nerve function, presence of lateralizing signs, and peripheral nerve exam, including documentation of motor/sensory levels. If it can be safely done, the neurological exam is especially important to perform prior to analgesia, sedating medications, or intubation. Finally, head-to-toe examination of the patient is done in a manner that exposes the patient while preventing hypothermia. Once the primary survey is complete, a secondary survey is carried out, and appropriate radiologic and laboratory investigations are ordered. These may include, but are not limited to: cervical spine, chest and pelvic X-rays, diagnostic peritoneal lavage, or focused assessment sonograph trauma ultrasound for suspected intra-abdominal injuries; computed tomography (CT) scan or angiography; specific extremity X-rays; and laboratory tests, including hematologic and chemistry profiles, arterial blood gas, cross-match, and toxicology screen.
| Eye opening | Spontaneously | 4 |
| To speech | 3 | |
| To pain | 2 | |
| None | 1 | |
| Verbal response | Oriented | 5 |
| Confused | 4 | |
| Inappropriate | 3 | |
| Incomprehensible | 2 | |
| None | 1 | |
| None – intubated | 1T | |
| Motor response | Obeys commands | 6 |
| Localizes to pain | 5 | |
| Withdraws from pain | 4 | |
| Flexion to pain (decorticate) | 3 | |
| Extension to pain (decerebrate) | 2 | |
| None | 1 | |
| Maximum score | 15 | |
| Minimum score | 3 (3T if intubated) |
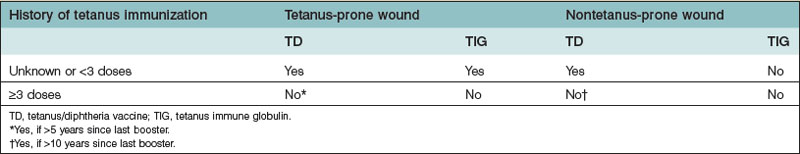
Concerning lower extremity injuries, it is not only important to assess the injury, but also to be aware of its associated complications, including significant bleeding from long-bone injuries, rhabdomyolysis from crush injury, fat embolization, and acute compartment syndrome. In the initial management of these injuries, splinting helps to control blood loss and reduce pain. During the secondary survey, an appropriate history helps determine the mechanism of injury and anticipated degree of injury. The physical examination of the lower extremity includes examination of the skin, neurological status, circulatory status, and skeletal or ligamentous injury. The degree of soft-tissue damage and contamination is important, as these patients will often require prophylactic broad-spectrum antibiotic coverage and tetanus immunization if not up to date (Table 2.2). Neurological examination should include sensory, motor function, and deep tendon reflexes. Assessment of posterior tibial nerve function can be particularly important in guiding decisions regarding limb salvage. Any circulatory compromise suggests possible arterial injury. Initial management should include fracture reduction, and if pulses are still not restored, appropriate angiographic studies should be sought along with consultation of an orthopedic or vascular surgery colleague. Vascular injury should be suspected in cases of active hemorrhage, expanding or pulsatile hematoma, thrill/bruit over wound, absent distal pulses, or distal ischemic manifestations (the five “P’s”: pain, pallor, paralysis, paresthesia, poikilothermy).22 Ischemic time should be estimated for any amputated or vascular-compromised segments. X-rays should be obtained of all areas of suspected injury, including the joint above and below the injury site.
Several grading systems exist to classify lower extremity trauma. The systems most commonly used are the Gustilo and the Byrd systems, in which tibial fractures are classified based on the size of the wound, amount of soft-tissue injury, bony injury, and presence of vascular injury (Table 2.3).23,24
Table 2.3 Classification of lower extremity injury
| System | Grade | Details |
|---|---|---|
| Gustilo | I | |
| II | ||
| III | ||
| IIIA | ||
| IIIB | ||
| IIIC | ||
| Byrd | Type I | |
| Type II | ||
| Type III | ||
| Type IV |
Patient selection
When managing the patient with complex lower extremity trauma, it is important to recognize that futile attempts at limb salvage may be associated with physical, mental, social, and financial implications.25 Despite advances in microsurgical reconstruction, bony regeneration, and infection control, many patients undergo a number of surgeries only to have eventual amputation. It is important to try and identify such patients at the outset, and provide the patient with the best option that maximizes function and recovery.
Amputation versus salvage
The establishment of the MESS by Johansen et al. in 1990 was the first effort to produce a set of guidelines that would help a physician to decide between salvage and amputation.26 This score relied on four criteria: (1) skeletal/soft-tissue injury; (2) limb ischemia; (3) shock; and (4) patient age (Table 2.4). A score of 7 or greater was used as the cutoff point in favor of amputation. Unfortunately, even with this tool there was still debate over definitive criteria for amputation, and this led to the recent multicenter study entitled the Lower Extremity Assessment Project (LEAP), carried out at eight level I trauma centers in the US. This study looked prospectively at patients with traumatic amputations of the lower leg, Gustilo III (A–C) injuries, lower leg devascularizing injuries, major soft-tissue injuries of the lower leg, open pilon fractures (grade III) and ankle fractures (grade IIIB), and severe open hindfoot and midfoot injuries with degloving and nerve injury.27 The goal of the study was to define the characteristics of the individuals sustaining lower extremity trauma. A total of 601 patients were enrolled, and the patient demographic was primarily male (77%), Caucasian (72%), and young (71% between 20 and 45 years old). The results demonstrated that the patients who sustained a high degree of extremity trauma had several disadvantages prior to their injury (social, economic, personality), and that quality of life and functional outcome data seemed more related to these than to the injury.
Table 2.4 Mangled Extremity Severity Score (MESS) criteria
| Variable | Points | |
|---|---|---|
| A | Skeletal/soft-tissue injury | |
| Low-energy (stab, simple fracture, civilian gunshot wound) | 1 | |
| Medium-energy (open/multiple fractures, dislocation) | 2 | |
| High-energy (close-range shotgun, military gunshot wound, crush) | 3 | |
| Very-high-energy (above + gross contamination) | 4 | |
| B | Limb ischemia* | |
| Pulse reduced or absent; perfusion normal | 1 | |
| Pulseless, paresthesias, diminished capillary refill | 2 | |
| Cool, paralyzed, insensate, numb | 3 | |
| C | Shock | |
| Systolic blood pressure always >90 mmHg | 1 | |
| Transient hypotension | 2 | |
| Persistent hypotension | 3 | |
| D | Age | |
| <30 years | 1 | |
| 30–50 years | 2 | |
| >50 years | 3 | |
| Maximum score possible | 16 | |
| Threshold score for amputation | 7 | |
* Score doubled for ischemia time >6 hours.
A subset of the LEAP studies assessed several available clinical decision-making scores relating to injury severity. These include MESS, LSI, PSI, NISSSA score, and HFS-97. When applying these scores to the open tibial fracture group, MESS, PSI, and LSI demonstrated high specificity (91%, 87%, 97% respectively) but low sensitivity (46% each).28 Specificity is important to insure that a minimal number of salvageable limbs are incorrectly assigned to amputation, and sensitivity is important to guard against delay in amputation for limbs that are not salvageable. Discrimination was moderately good in assessing salvage versus amputation of the limb. Overall, the analysis did not validate the clinical utility of any of the lower extremity injury severity scores, and advised for their cautious application when making decisions regarding limb salvage. Additionally, these scores are not predictive of functional recovery in patients who undergo successful limb reconstruction, as shown by Ly et al.29
Unfortunately, despite a number of studies, there are still no definite criteria for amputation. Several proposed criteria have since been refuted following proper outcome studies. For example, it is a widely held belief that tibial nerve injury or absence of plantar foot sensation is an indication for amputation. However, in a study by Bosse et al.30 examining functional outcomes of patients with severe lower extremity injuries, they found that more than half of the patients who initially presented with an insensate foot that underwent salvage regained sensation by 2 years. The authors concluded that initial plantar sensation was not prognostic of long-term plantar sensation status or functional outcome, and that this should therefore not be a criterion in limb salvage algorithms.
Risk factors that may contribute to or predict the need for amputation include22:
• Gustilo IIIC tibial injuries
• sciatic or tibial nerve injury
• prolonged ischemia (>4–6 hours)/muscle necrosis
• crush or destructive soft-tissue injury
• significant wound contamination
• multiple/severely comminuted fractures; segmental bone loss
• old age or severe comorbidity
• apparent futility of revascularization or failed revascularization.
In addition to these risk factors, several prognostic factors for limb salvage have been identified.22 These include: mechanism of injury, anatomy of injury (e.g., popliteal artery injury has worst prognosis), presence of associated injuries, age and physiologic health of the patient, and clinical presentation (e.g., shock, limb ischemia). The environmental circumstance may also play a role in determining salvage, with higher amputation rates in combat zones, austere environments, and multicasualty events.
Treatment and surgical techniques
Timing of treatment
There is no debate that a patient with complex trauma should undergo thorough irrigation of open fractures and debridement of devitalized tissue. This is considered to be of paramount importance for the prevention of infection. It is commonly taught that the standard of care is to take patients to the operating room within 6 hours of injury.31,32 This timeframe is not supported by evidence, and was recently addressed in a subgroup analysis of the LEAP study. Among those patients who developed major infections, significant factors included bone loss >2 cm and Gustilo-type IIIC fractures. Factors such as degree of nerve/muscle damage, size of skin defect, injury severity score, and surgeon’s assessment of the degree of contamination were nonpredictive of infection. Regarding their treatment, patients who were managed with intramedullary (IM) nail had a lower incidence of infection compared with those who were externally fixated or treated with plate/screw fixation. There was no significant difference in terms of mean time to debridement between patients who developed infection and those who did not. Time from initial debridement to eventual soft-tissue coverage was also not a risk factor for infection. Multivariate regression showed that patients with delayed admission to a primary trauma center (>2 hours postinjury) or delayed transfer from an outside institution to primary trauma center resulted in higher rates of infection.33 It is unclear whether delay in admission to a trauma center was in fact a surrogate marker indicative of a higher degree of injury.
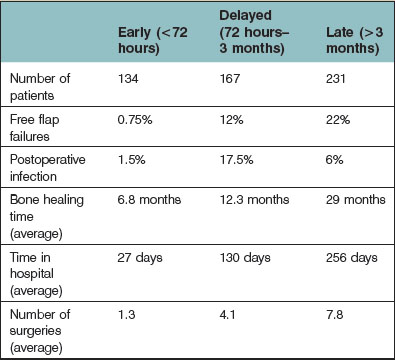
With regard to the timing of reconstruction of lower-extremity trauma, most believe that early reconstruction leads to better outcome. This follows the study by Godina34 in which the timing of microsurgical reconstruction was analyzed with respect to flap failure, infection, bone-healing time, length of hospital stay, and number of operative procedures. This study stratified patients into three groups of repair: (1) within 72 hours; (2) between 72 hours and 3 months; and (3) beyond 3 months postinjury. The study concluded that reconstruction within 72 hours showed the best outcomes for all analyzed factors. Those reconstructed between 72 hours and 3 months postinjury had the highest flap failure rates and highest rates of infection. Those reconstructed beyond 3 months had the longest time to bone healing, and the greatest number of operations (Table 2.5). Criticisms of Godina’s study include the fact that the initial 100 patients in the series were treated in a delayed fashion with average operative times of 6–8 hours, whereas those subsequently treated in an immediate fashion (when microsurgical experience was improved) had shorter operative times.35 Patients were also not randomized to their treatment regimes, had variable reconstruction (i.e., different flap choices), and upper/lower extremity reconstruction outcomes were pooled. There have been several subsequent studies on this topic – and the results have varied from indifference to favoritism of immediate reconstruction.23,35–39 Again, the majority of these have been retrospective reviews, where patients were not randomized to treatment, with nonstandardized reconstructive options.
Finally, it is important to recognize that immediate reconstruction is not an option for every patient – and relies on a surgeon capable of performing single-stage debridement and immediate reconstruction, a stable patient without other life-threatening injuries.40 In this case, serial debridement and possibly the use of wound vacuum-assisted closure dressings may be optimal.
Fracture management
The initial orthopedic management of an open lower extremity fracture has traditionally involved external fixation, in an effort to avoid implantation of metal in a contaminated field. Recently, more orthopedic surgeons are choosing to fixate fractures acutely using IM nails, or even plates and screws using a technique called minimally invasive plate osteosynthesis.40 The safety of an unreamed IM nail is thought to be equivalent to external fixation with respect to union, delayed union, deep infection, and chronic osteomyelitis.41 Use of external fixators may be associated with pin tract infection, and may interfere with access for microvascular reconstruction.
Vascular injury
True vascular injury in the setting of lower extremity trauma is rare. Most definitive signs of vascular compromise can be attributed to soft-tissue and bone bleeding, traction of intact arteries with pulse loss (i.e., due to displaced fractures), or compartment syndrome. However, it is prudent to rule out vascular injury, and therefore liberal use of imaging should be carried out in the presence of hard signs. Traditionally, the arteriogram was the gold standard in the diagnosis of vascular injuries. Although it is accurate in diagnosing vascular injury, it has several disadvantages, including cost, duration of procedure, and potential delay to repair, and need for a specialized team to perform it.42 There is also an associated risk of morbidity in the form of contrast allergy and percutaneous vascular access-related complications. The advent of CT angiography (CTA) has helped to provide rapid diagnosis of vascular injury with lower morbidity. It also enables multiple parts of the body to be assessed. CT angiographic signs of arterial injury include active extravasation of contrast material, pseudoaneurysm formation, abrupt narrowing of an artery, loss of opacification of an arterial segment, and arteriovenous fistula formation.42 Studies comparing CTA and conventional angiography have demonstrated a high sensitivity and specificity of CTA for arterial injury that was confirmed with conventional angiography. There is also a significant cost saving – in one institution savings exceeded $13 000 per patient when performing CTA alone.43
There is no doubt that “time is muscle,” and prompt repair of any vascular injury should be carried out. Permanent ischemic injury may occur anywhere from 2 to 12 hours postinjury according to the literature. This wide range may be due to variation in injury mechanism, presence of collateral flow, and level of injury.44 The decision to perform a definitive vascular repair in the setting of lower extremity trauma depends on the clinical circumstances. There are some situations in which the patient is best served with temporary intraluminal shunting following distal thrombectomy and heparinization. These include: unstable fractures, gross contamination of the wound bed, soft-tissue deficits that would lead to an exposed reconstruction/repair, unstable patient, unavailable resources/surgical expertise in vascular repair, and coincident life-threatening injury.22
In combined injuries involving bone and vascular disruption, the operative sequence of repair has been debated. In one systematic review, survival of the extremity was directly related to ischemic time, with a steep increase in incidence beyond 3–4 hours of ischemic time.45 Those favoring immediate vascular repair believe that the reversal of ischemia is most important, whereas those favoring skeletal fixation prior to vascular repair argue that stabilization of the bony fragments first will avoid disruption of a vascular repair during bony manipulation. There have been several reports of fracture fixation following vascular repair, with no disruption of the repair.46–48 A meta-analysis comparing the outcomes of surgical sequence in 14 published studies did not demonstrate a clear difference between groups regarding incidence of subsequent amputation.49
Reconstructive options
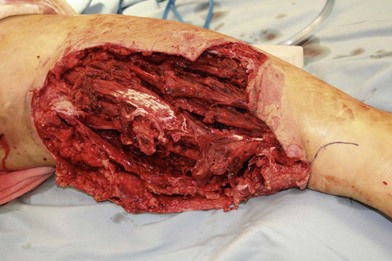
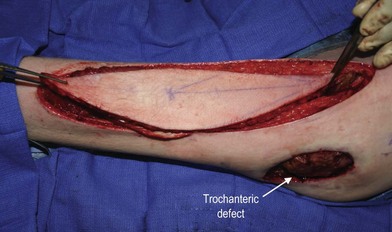
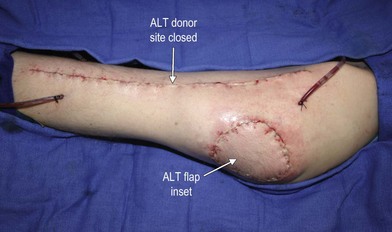
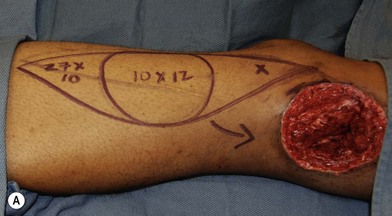
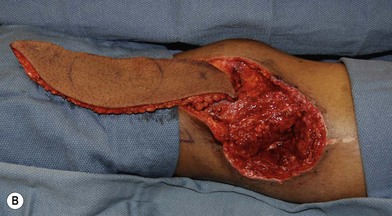
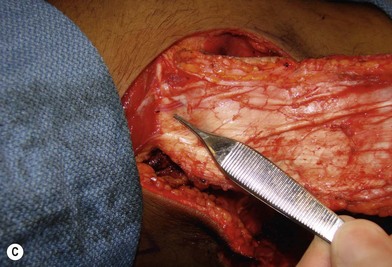
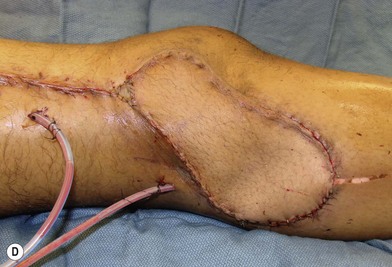
In patients with lower extremity trauma, it is important to consider all options for reconstruction, and choose that which is most reliable or will provide the best functional outcome. The reconstructive ladder is often considered, but in major lower extremity trauma where soft-tissue damage is extensive and the zone of injury is wide, local options are often eliminated, emphasizing the need for free flap coverage. When choosing a reconstructive option, consideration is given to the size and location of the defect, functional needs (i.e., innervated flap), zone of injury, proposed location of vascular anastomosis for free flap reconstruction, and accordingly the length of vascular pedicle required to achieve this. Consideration should also be given to the need for future operative interventions (e.g., tendon reconstruction, bony reconstruction), and flap choice should consider ease of re-elevation. Regardless of flap options chosen for reconstruction, adequate debridement of all nonviable tissue is imperative. High-energy trauma can result in significant soft-tissue destruction with a need for extensive debridement of nonviable tissue (Fig. 2.1).
Local flaps
There are a variety of local flap options available in lower-extremity reconstruction. Based on anatomical and angiographic studies, local flaps may be safely performed when based on known perforators. Local flaps are advantageous as they offer a viable reconstructive option with shorter operative times and less complexity.50 They also provide tissue that is similar in composition to the area of the defect. Patients should be screened for comorbidities that would compromise vascular supply of the lower extremity, and have an appropriate vascular exam with or without investigations performed. A number of local flap options are discussed in the following sections on perforator flaps and muscle-based flaps.
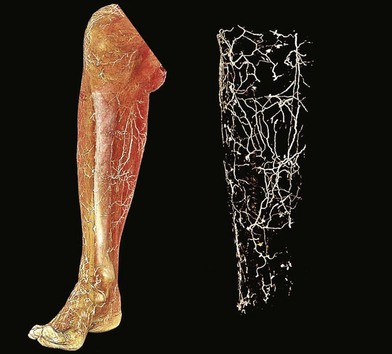
Perforator flaps
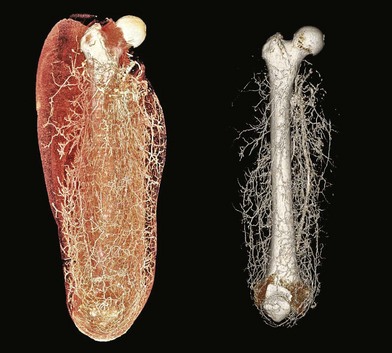
The lower extremity is an anatomic region rich in vascular perforators available for harvest (Figs 2.2 and 2.3). These have been mapped out in extensive detail through cadaveric injection studies and angiographic investigations. In the gluteal region, the majority of the perforators are musculocutaneous, and arise from the superior and inferior gluteal arteries, with minor contributions from the iliolumbar artery and internal pudendal artery. These are the basis for commonly used gluteal perforator flaps including the superior and inferior gluteal artery perforator flaps.
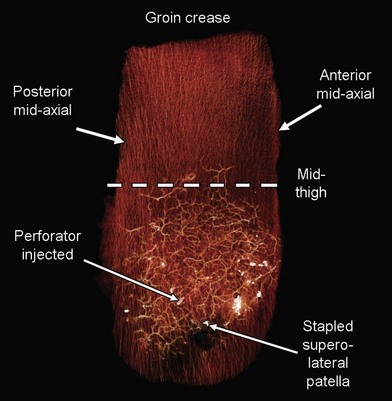
In the hip and thigh region there are a combination of musculocutaneous and septocutaneous perforators from six source arteries originating from the common femoral artery.51 The anteromedial thigh is supplied by femoral artery perforators and superficial femoral artery perforators that supply the anteromedial thigh perforator flap. The anterolateral thigh (ALT) is primarily supplied by the lateral circumflex femoral artery (ascending, transverse, and descending branches). These perforators supply the tensor fasciae latae perforator flap, and the ALT (Figs 2.4 and 2.5). Just superior to this is the superficial circumflex iliac artery, which provides perforators to the free superficial circumflex iliac artery perforator flap, and contributes to the groin flap. Perforators from the medial circumflex femoral, profunda femoris, and popliteal arteries supply the posteromedial thigh. The posterolateral thigh is supplied by the profunda femoris and inferior gluteal arteries. Inferiorly, the medial and lateral superior genicular arteries supply the region around the knee, and are a source of local perforator flaps in knee reconstruction (Figs 2.6, 2.7).