21 Management of facial burns
Synopsis
 Early, accurate diagnosis with early aggressive therapy of the acute burn wound is one of the keys to optimal final result, and is the beginning of restoration after facial burn injury.
Early, accurate diagnosis with early aggressive therapy of the acute burn wound is one of the keys to optimal final result, and is the beginning of restoration after facial burn injury.
 Nonoperative therapy to allow optimal natural healing and scar maturation is important to minimize the extent of reconstructive surgery and optimize the final result.
Nonoperative therapy to allow optimal natural healing and scar maturation is important to minimize the extent of reconstructive surgery and optimize the final result.
 Reconstructive surgery should be delayed until substantial burn scar maturation has occurred.
Reconstructive surgery should be delayed until substantial burn scar maturation has occurred.
 Reconstructive surgery for the late effects of burn injury is set apart from other reconstructive surgery by the exceedingly common lack of local normal skin for donor sites.
Reconstructive surgery for the late effects of burn injury is set apart from other reconstructive surgery by the exceedingly common lack of local normal skin for donor sites.
 Specific techniques most commonly used in facial burn reconstruction practice are described in detail with emphasis on important
Specific techniques most commonly used in facial burn reconstruction practice are described in detail with emphasis on important
 An algorithm can be used to analyze facial burn scar deformities and help make decisions regarding reconstruction.
An algorithm can be used to analyze facial burn scar deformities and help make decisions regarding reconstruction.
 Operatively, flaps from local donor sites should be used whenever possible.
Operatively, flaps from local donor sites should be used whenever possible.
 Technical aspects of burn reconstruction unique to specific anatomic areas are described.
Technical aspects of burn reconstruction unique to specific anatomic areas are described.
 Once the reconstructive surgery operation is complete, non-operative therapy of the new surgical scars should commence.
Once the reconstructive surgery operation is complete, non-operative therapy of the new surgical scars should commence.
 Secondary operations are common, and frequently follow placement of a large amount of tissue for contracture release or resurfacing to provide the optimal result.
Secondary operations are common, and frequently follow placement of a large amount of tissue for contracture release or resurfacing to provide the optimal result.
Introduction
Annually, there are more than 500 000 burns treated in the US. A total of 40 000 patients are hospitalized and 25 000 are hospitalized in specialized burn centers. There are 4000 fire and burn deaths per year in the US. The most common causes of burn injury are fire/flame (46%) and scald (32%), with scald burns being a particular problem in children.1
History
The development of the parachute subsequently led to an increasing number of airmen who parachuted to safety after sustaining severe burn injuries in their burning planes in the Second World War. These injuries along with burns from other war-related explosions provided the first large experience with facial burn injuries. In 1930, Gillies invited his cousin, Sir Archibald McIndoe to join his practice. McIndoe continued and furthered the work of Gillies and, in 1941, following the Battle of Britain, while the real treatment of burns was still in its infancy, began his pioneering reconstructive operations on facial burn scars elaborating further principles specifically directed to the approach to facial burn scarring.2
Although tissue expansion was first introduced by Neumann in 1957,3 Radovan popularized it with its use for breast reconstruction in the late 1970s and early 1980s.4 In burn reconstruction, expanded advancement flaps began being used for resurfacing after excision of adjacent burn scars. The idea of expanding normal skin to provide more donor site for burn reconstruction was introduced.5 Tissue expanded flaps were found to be more vascularized than normal skin, and flaps were found to survive for greater lengths.6,7 This ultimately led to increased availability of better suited skin for facial reconstruction, expanded free flaps and pre-fabricated flaps.
Basic science
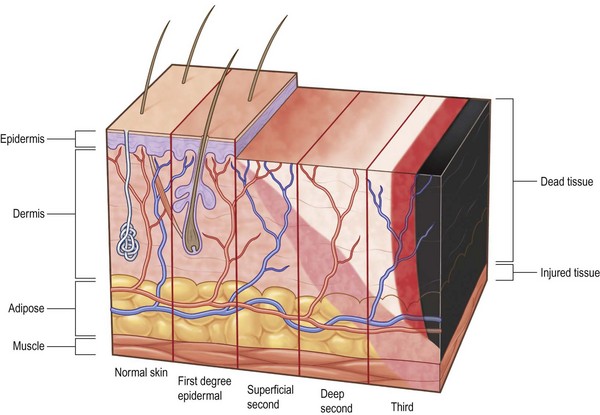
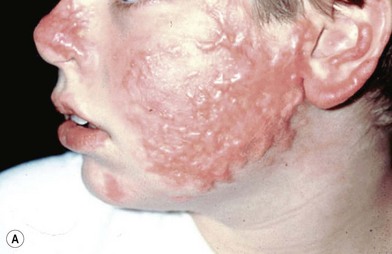
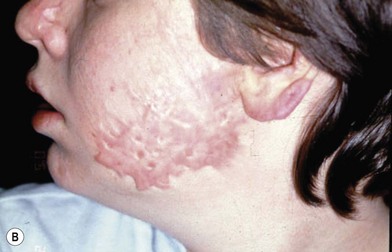
The degree of tissue damage is classified by the depth of the damage to the skin and the underlying tissues (Fig. 21.1). Damage to the epidermal layer alone is described as an epidermal burn scientifically, and a first-degree burn in common usage. An example of a first-degree burn is typical sunburn. An epidermal burn heals by sloughing the dead epidermal cells with immediate regrowth of new cells.
Diagnosis: determination of depth of burn
Cole et al. have proposed that the decision to operate on indeterminate facial dermal burns be made at approximately 10 days, and based on the best clinical judgment, excise if indicated and graft with thick split-thickness skin graft.8
Recently the scanning laser Doppler has been shown to help with the diagnosis in indeterminate dermal burns.9–11 This device determines the relative amount of blood flow in the superficial burn wound by reading the change in frequency of laser light caused by the movement of blood cells within the wound as it scans the surface of the wound. The color and numerical flux readings correspond to the likelihood of the burn wound healing within the 2–3 weeks window consistent with healing by re-epithelialization and minimal or no scarring.
Treatment of acute facial burns
Because of the high degree of vascularization of the facial skin, infection of facial burn wounds is less likely than burns in other anatomic areas. This allows for some delay in making critical decisions regarding excision and grafting of facial burns. For management of the nonfacial burn, the idea of early tangential excision of deep dermal and full-thickness burns was championed by Janzekovic in the 1970s and continues to be the standard of care. Controversy still exists among surgeons regarding the early excision and grafting of facial burns. It is currently recommended to first treat a deep burn nonoperatively for 7–10 days with topical ointments, creams and local debridement.8,12 Some deep burns are obvious and may be excised and grafted earlier, but the vast majority are initially indeterminate.
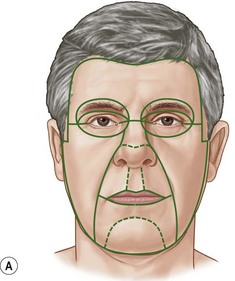
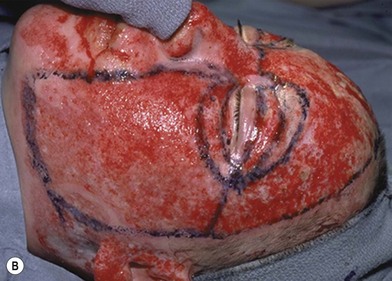
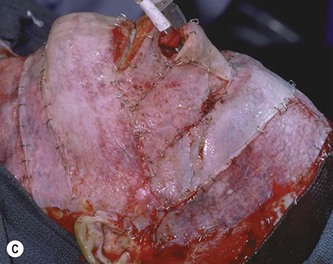
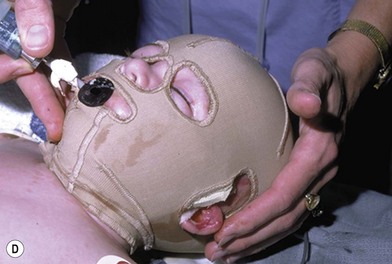
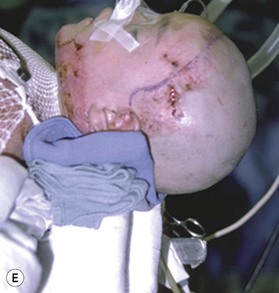
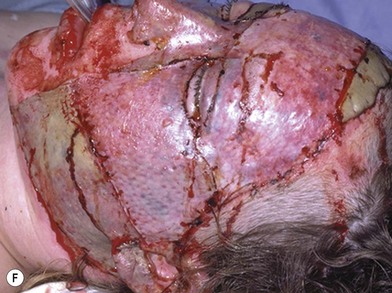
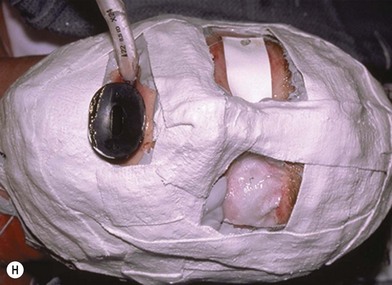
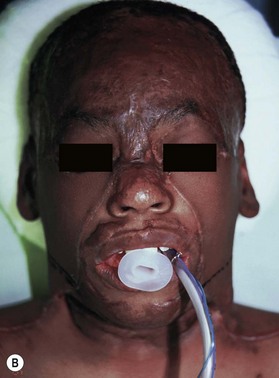
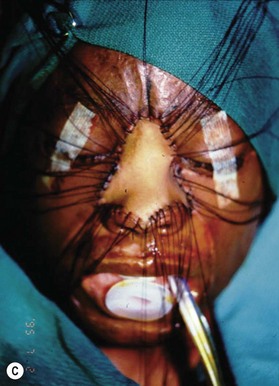
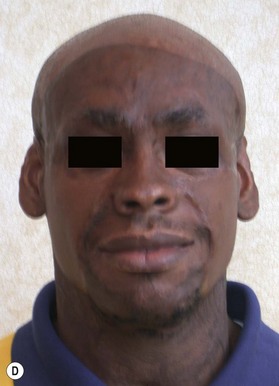
Deep facial burn injuries that will clearly not heal by epithelialization are doomed to heal with hypertrophic scarring and contractures if there is no surgical intervention. The standard surgical intervention is early excision and grafting in facial aesthetic units. This should be done as early as possible to minimize the migration of fibroblasts into the facial burn wound. Engrav recommends that a decision be made to excise and graft by 7–10 days post-injury, and have the face entirely grafted by 21 days. Initial excision of the wound is followed by sheet split-thickness skin allograft for 1 week. After that, the patient is returned to the operating room for closure with thick split-thickness autografts in the range of 0.018–0.021 inches in adults, and 0.008–0.012 inches in children placed in facial aesthetic units (Fig. 21.2).12
Eyes/eyelid
Generally, it is rare for a thermal injury to involve the globe. In the current literature, the reported incidence of ocular, intraocular, intraorbital foreign bodies (especially in explosions) and periorbital injury due to a burn ranges from 8% to 20% of all burn admissions.13 This is most likely due to a significant number of protective mechanisms that include a patient’s reflex protective movements of the head and arms, and more importantly, the blink reflex. It should be noted that one of the most commonly overlooked or missed foreign body is the contact lens.
Most commonly the problem is globe protection in burns that damage the eyelids. Various ointments, corneal shields, or protective contact lenses help shield the cornea from further injury. A temporary suture tarsorrhaphy may be useful and effective in aiding lid closure, but dehiscence and complications are common, making tarsorrhaphy use controversial.14 Tarsorrhaphy does not prevent wound contraction, and the basic principles of early excision and grafting, and ectropion release and tissue transplantation are primary.
Wound nonoperative management of facial burn scars
Once facial burn wounds are healed, there are a number of ways that facial scars can be minimized and maturation of the scars can be encouraged. The two primary mainstays of non-operative facial burn scar therapy are compression and contact with silicone gel. The silicone gel is thought to be effective in reducing scarring and in encouraging scar maturation.15,16 Compression tends to keep the hypertrophy of the scars in check.
The use of pulsed dye laser therapy has been used to reduce the erythema and pruritus, and possibly improve scar remodelling in immature hypertrophic burn scars.17,18 Carbon dioxide fractional ablative laser therapy seems to have a positive effect on remodeling of mature burn scars sometimes many years after forming.19,20
Surgical management of the late effects of face, head and neck burns
General principles
Importance of allowing total wound healing and scar maturation
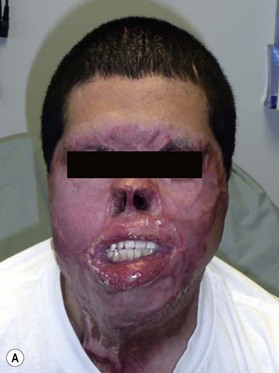
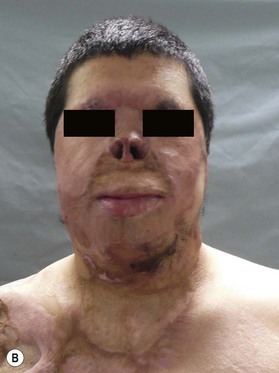
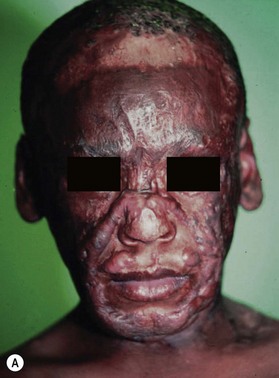
Additionally, the time waiting for reconstruction frequently results in resolution of erythema and hypertrophy of some areas of scar, and, in some cases, improving function through therapy resulting in a reduction of the extent of the scar problem requiring surgical intervention (Fig. 21.3).
Reconstruction must be preceded by analysis of the problem and diagnosis of the factors causing it
Do not assume that the entire scar must be treated similarly. Another element in diagnosis is determining whether treating only a portion of the scar will provide the desired improvement while leaving another area of the scar untreated. This is particularly true in determining the tension in a scar. By releasing tension, hypertrophy which is frequently potentiated by the tension is minimized in the rest of the scar.21
Restoration of function usually precedes aesthetic reconstruction
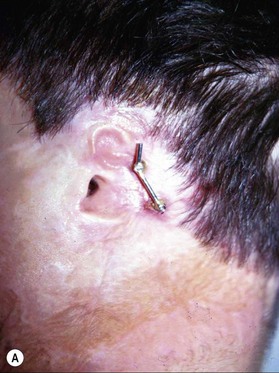
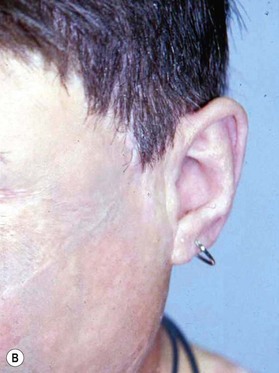
Release of contracture usually is the first priority. Extrinsic contractures, contractures of the neck in the case of facial burn scars, need to be released before the true extent of facial intrinsic contractures can be determined (Fig. 21.4). Neck contracture release also allows for safer anesthetic management by allowing neck extension.
Skin grafts are used because of their thinness when a thicker flap would hide underlying anatomic contours or interfere with function
Skin grafts are less stable in their characteristics. They change color and other characteristics unpredictably, but starting with the best well-matched local skin available gives the best chance of success.22
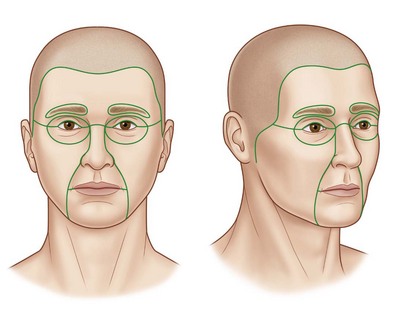
Skin replacement should generally be performed in “aesthetic units” when subtotal or total unit defects are present
This avoids the aesthetically23 unfavorable appearance of a ‘patch.’ Complete excision of the entire aesthetic unit including residual normal skin should, at least, be considered if more than half the aesthetic unit is scar (Fig. 21.5).
Skin replacement should be performed with as well-matched skin as possible to the recipient site
The best donor sites matching facial skin come from the upper chest, shoulders, and scalp, commonly called the ‘blush areas.’22
Ancillary techniques should be employed to maximum advantage
The ancillary techniques are numerous and include cosmetics, tattooing, hair transplantation, prosthetics (Fig. 21.6), laser therapy and others (Box 21.1).
An algorithmic approach to facial burn in reconstruction
Based on some of the above general principles, a framework (Fig. 21.7) evolved in the author’s practice allows for analyzing burn-related facial deformity and dysfunction, simplifying often complex problems, and guiding reconstructive surgical decisions.24,25
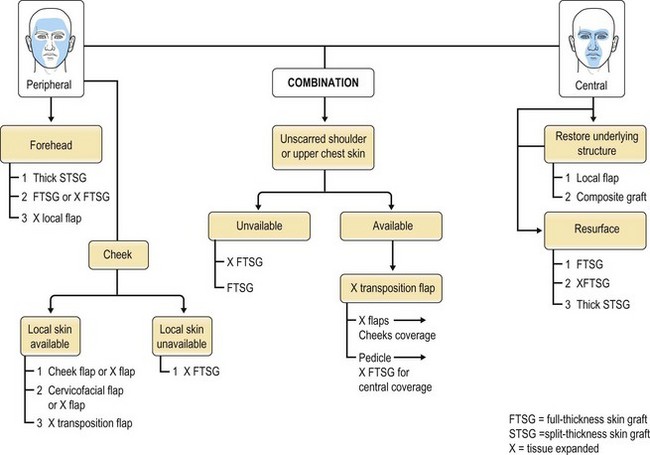
Fig. 21.7 Algorithm for the reconstruction of large facial deformities.
(From Spence RJ. An agorithm for total and subtotal facial reconstruction us an expanded transposition flap: a 20-year experience. Plast Reconstr Surg. 2008;121(3):795–805.)
This algorithm also incorporates all available techniques ranging from the simplest scar revision or excision and closure to complete replacement of the skin of the face. When scarred skin replacement is indicated, the algorithm recommends flap replacement of the peripheral aesthetic units as these areas are best reconstructed with broad, relatively featureless normal skin. However, when the replacement of the central facial aesthetic units is indicated, the infrastructure of the various contours needs to be reconstructed first, if necessary, and covered with a thin skin replacement usually in the form of full-thickness skin graft. As described in the literature, the expanded transposition flap26 is central in the algorithm when major facial deformities are addressed, as it provides both relatively thin flaps for the peripheral aesthetic units and full-thickness skin grafts for the central aesthetic units (Fig. 21.8).
Specific techniques
Scar replacement with local skin
The simplest example of scar replacement with local skin is simple scar excision with primary closure. This excises the burn scar and replaces it with a surgical scar that is designed to be better and, optimally, much less noticeable. Basic plastic surgery principles critical to obtaining the best results include: (1) performing the excision within relative adjacent tissue excess where possible; and (2) respecting resting skin tension lines (RSTL) in the planning of the new scar (Fig. 21.9).27 This repositions the new scar in a pre-existent anatomic line or contour such as the nasolabial crease, a hairline, or forehead wrinkles. It also imparts the least tension across the new scar minimizing the negative effects of tension on the scar.
Serial excision
An extension of simple scar replacement by excision and primary closure is the technique of serial excision. When a scar is too large for excision and primary closure, only that portion of the scar that can be excised is, and the wound closed. The tension resulting from the first wound closure results in stretching of the surrounding skin (mechanical creep) and subsequent growth of the skin in response to the additional tension (biologic creep).28 The increase in the amount of surrounding skin that takes place over at least 3 or 4 months allows reoperation to excise the residual scar and close the resulting wound with the expanded normal surrounding skin. With the advent of tissue expansion to develop new skin adjacent to scars, serial excision is used much less, but should be considered if the serial excision is likely to take only two or, at most, three operations.
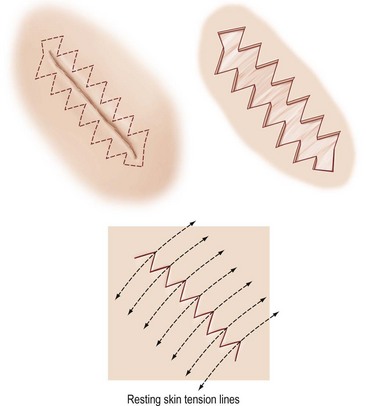
W-plasty
A W-plasty is the closure of a wound with multiple sawtooth lines that better approximate the resting skin tension lines (RSTL) of the facial skin (Fig. 21.10).29 The resulting lines more in-line with the RSTL become less noticeable. They also break up the linear nature of the unfavorable scar. A similar effect can occur if multiple small Z-plasties are placed in the linear skin closure. Technically, this technique can be exceedingly tedious. For W-plasty closure to be safe and effective, it needs to have limbs that are millimeters in length not to exceed 1 centimeter.