Lupus Erythematosus: Introduction
|
Lupus Erythematosus: A Challenge to Define, Classify, and Treat
Lupus erythematosus (LE) is the root designation for a diverse array of illnesses that are linked together by the development of autoimmunity directed predominantly at the molecular constituents of nucleosomes and ribonucleoproteins. Some patients present with life-threatening manifestations of systemic LE (SLE); whereas others, who are affected with what likely represents the same basic underlying disease process, express little more than discoid LE (DLE) skin lesions throughout their illness. It is convenient to conceptualize LE as a clinical spectrum ranging from mildly affected patients with only localized DLE skin lesions to those at risk of dying from the systemic manifestations of LE such as nephritis, central nervous system disease, or vasculitis. The pattern of skin involvement expressed by an individual patient with LE can provide insight about the position on the spectrum where the patient’s illness might best be placed.
The nomenclature and classification system originally devised by James N. Gilliam divides the cutaneous manifestations of LE into those lesions that show characteristic histologic changes of LE (LE-specific skin disease) and those that are not histopathologically distinct for LE and/or may be seen as a feature of another disease process (LE-nonspecific skin disease). Within this context, the term “LE-specific” relates to those lesions displaying an interface dermatitis. The term cutaneous LE (CLE) is often used synonymously with “LE-specific skin disease” as an umbrella designation for the three major categories of LE-specific skin disease: acute cutaneous LE (ACLE), subacute cutaneous LE (SCLE), and chronic cutaneous LE (CCLE). This will be the framework used in our discussion of the extraordinarily diverse set of cutaneous lesions that occur in patients with LE (Table 155-1).
LE-Specific Skin Disease [Cutaneous LE (CLE)] | LE-Nonspecific Skin Disease |
|---|---|
|
|
The essence of LE is in its heterogeneity, and the challenge for those who treat it is to recognize clinically useful patterns within the mosaic of features that constitute this protean illness. An overview of the systemic manifestations of LE can be seen in the American College of Rheumatology’s (ACR) classification criteria for SLE,1 which are presented in Table 155-2, and from the outline of the systemic manifestations of SLE presented in Table 155-3.
Criterion | Definition |
|---|---|
| 1. Malar rash | Fixed erythema, flat or raised, over the malar eminences, tending the spare the nasolabial folds |
| 2. Discoid rash | Erythematous raised patches with adherent keratotic scaling and follicular plugging; atrophic scarring may occur in older lesions |
| 3. Photosensitivity | Skin rash as a result of unusual reaction to sunlight, by patient history or physician observation |
| 4. Oral ulcers | Oral or nasopharyngeal ulceration, usually painless, observed by a physician |
| 5. Arthritis | Nonerosive arthritis involving two or more peripheral joints, characterized by tenderness, swelling, or effusion |
| 6. Serositis | a. Pleuritis—convincing history of pleuritic pain or rub heard by a physician or evidence of pleural effusion |
| Or | |
| b. Pericarditis—documented by electrocardiogram or rub or evidence of pericardial effusion | |
| 7. Renal disorder | a. Persistent proteinuria— >0.5 g/day or greater than 3+ if quantitation not performed |
| Or | |
| b. Cellular casts—may be red cell, hemoglobin, granular, tubular, or mixed | |
| 8. Neurologic disorder | a. Seizures—in the absence of offending drugs or known metabolic derangements (e.g., uremia, ketoacidosis, or electrolyte imbalance) |
| Or | |
| b. Psychosis—in the absence of offending drugs or known metabolic derangements (e.g., uremia, ketoacidosis, or electrolyte imbalance) | |
| 9. Hematologic disorder | a. Hemolytic anemia—with reticulocytosis |
| Or | |
| b. Leukopenia— <4,000 μL total on two or more occasions | |
| Or | |
| c. Lymphopenia— <1,500/μL on two or more occasions | |
| Or | |
| d. Thrombocytopenia— <100,000 μL in the absence of offending drugs | |
| 10. Immunologic disorder | a. Anti-DNA—antibody to native DNA in abnormal titer |
| Or | |
| b. Anti-Sm—presence of antibody to Sm nuclear antigen | |
| Or | |
| c. Positive finding of antiphospholipid antibodies based on (1) an abnormal serum level of immunoglobulin G or immunoglobulin M anticardiolipin antibodies, (2) a positive test result for lupus anticoagulant using a standard method, or (3) a false-positive serologic test for syphilis known to be positive for at least 6 months and confirmed by Treponema pallidum immobilization or fluorescent treponemal antibody absorption test | |
| 11. Antinuclear antibody | An abnormal titer of antinuclear antibody by immunofluorescence of an equivalent assay at any point in time and in the absence of drugs known to be associated with “drug-induced lupus” syndrome |
|
|
Epidemiology
The epidemiology and socioeconomic impact of LE in general,2 and CLE specifically,3 have been reviewed. Skin disease is the second most frequent clinical manifestation of LE after joint inflammation. As many as 45% of patients with CLE experience some degree of vocational handicap. A recent quality of life study suggested that the impact of skin manifestations in patients with SLE was preceded only by pain and fatigue related to their disease.4 The Dermatology Life Quality Index and SF-36 have been used to measure quality of life in patients with CLE. Both questionnaires showed that patients with active skin lesions had lower quality of life and that patients with associated alopecia were particularly impacted.5
Malar, or butterfly rash (localized ACLE), has been reported in 20%–60% of large cohorts of patients with LE. Limited data suggest that the maculopapular or SLE rash of generalized ACLE is present in about 35%–60% of patients with SLE. ACLE, like SLE in general, is much more common in women than men (8:1). All races are affected; however, the early clinical manifestations of ACLE can be overlooked in a dark-skinned individual.
Patients presenting with SCLE lesions constitute 7%–27% of LE patient populations. SCLE is primarily a disease of white females, with the mean age of onset in the fifth decade. Drug-induced SCLE patients are somewhat older at disease onset, perhaps reflecting greater exposure to drugs for age-related medical problems (hypertension, cardiovascular disease).
The most common form of CCLE, a classic DLE skin lesion, is present in 15%–30% of SLE populations selected in various ways. Approximately 5% of patients presenting with isolated localized DLE subsequently develop SLE. Rheumatologists have estimated that SLE patients are sevenfold more common than isolated cutaneous LE patients. However, dermatologists have estimated that isolated cutaneous LE patients may be 2-3 times more common that SLE patients. Recently published population-based data have argued strongly that the incidence and prevalence of isolated forms of cutaneous LE are equivalent to those of SLE.6 Although DLE can occur in infants and the elderly, it is most common in individuals between 20 and 40 years of age. DLE has a female-male ratio of 3:2 to 3:1, which is much lower than that of SLE. All races are affected, but investigations suggest that DLE might be more prevalent in blacks.
Etiology and Pathogenesis
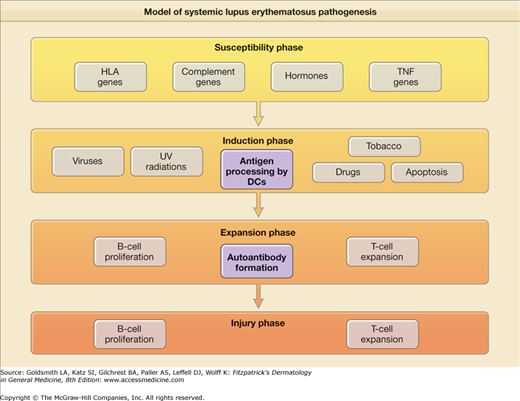
The cause(s) of and pathogenetic mechanisms responsible for LE-specific skin disease are not fully understood, although recent work has provided many new insights. The pathogenesis of LE-specific skin disease is inextricably intertwined with SLE pathogenesis. Simply put, SLE is a disorder in which the interplay between host factors (susceptibility genes, hormonal milieu, etc.) and environmental factors [ultraviolet (UV) radiation, viruses, and drugs] leads to loss of self-tolerance, and induction of autoimmunity. This is followed by activation and expansion of the immune system, and eventuates in immunologic injury to end organs and clinical expression of disease7 (eFig. 155-0.1). Recent work has highlighted the important role of interferon-α signaling in the pathogenesis of both SLE and LE-specific skin disease.
![]() The genetic association between LE-specific skin disease and serologically determined major histocompatibility complex (MHC) class II DR specificities is well known. For example, patients with SCLE and overlapping features of Sjögren’s syndrome are more likely to have the HLA-DR3, DQA1*0501, DQB1*0502 haplotype. Other susceptibility genes outside the MHC region have also been implicated. These include interleukin 1 (IL-1) receptor antagonist and tumor necrosis factor-α (TNF-α). Werth and colleagues8 found a substantial increase in the prevalence of a promoter polymorphism of TNF-α (-308A) in patients with SCLE. TNF-α may be a marker allele for the extended HLA-A01,B08, DRB1*0301 haplotype that is associated with a number of autoimmune conditions, including SCLE.9 Genetic deficiency of the early complement components C1q, C4, and to a lesser extent, C2, predispose patients to the development of lupus.10 In fact, complete congenital deficiency of C1q is the strongest single genetic risk factor yet identified for the development of photosensitive SLE.11
The genetic association between LE-specific skin disease and serologically determined major histocompatibility complex (MHC) class II DR specificities is well known. For example, patients with SCLE and overlapping features of Sjögren’s syndrome are more likely to have the HLA-DR3, DQA1*0501, DQB1*0502 haplotype. Other susceptibility genes outside the MHC region have also been implicated. These include interleukin 1 (IL-1) receptor antagonist and tumor necrosis factor-α (TNF-α). Werth and colleagues8 found a substantial increase in the prevalence of a promoter polymorphism of TNF-α (-308A) in patients with SCLE. TNF-α may be a marker allele for the extended HLA-A01,B08, DRB1*0301 haplotype that is associated with a number of autoimmune conditions, including SCLE.9 Genetic deficiency of the early complement components C1q, C4, and to a lesser extent, C2, predispose patients to the development of lupus.10 In fact, complete congenital deficiency of C1q is the strongest single genetic risk factor yet identified for the development of photosensitive SLE.11
![]() One of the most striking clinical observations in SLE is the disease’s female predilection. Indeed, there is a lifetime female-male ratio of 9:1. This sexually dimorphic prevalence is likely due to the effect of sex hormones on the immune system. High levels of estrogen have been shown to cause patients with SLE to have an increase in (1) the number of self-reactive lymphocytes that bypass developmental deletion, (2) an increase in CD4/CD8 ratio (favoring humoral responsiveness), and (3) the number of B cells leaving the bone marrow that express high-affinity recognition of self-DNA.12
One of the most striking clinical observations in SLE is the disease’s female predilection. Indeed, there is a lifetime female-male ratio of 9:1. This sexually dimorphic prevalence is likely due to the effect of sex hormones on the immune system. High levels of estrogen have been shown to cause patients with SLE to have an increase in (1) the number of self-reactive lymphocytes that bypass developmental deletion, (2) an increase in CD4/CD8 ratio (favoring humoral responsiveness), and (3) the number of B cells leaving the bone marrow that express high-affinity recognition of self-DNA.12
Genetic predisposition for a lupus diathesis does not, in itself, produce disease. Rather, it appears that induction of autoimmunity in such patients is triggered by some inciting event, likely an environmental exposure. Drugs, viruses, UV light, and, possibly, tobacco, have been shown to induce development of SLE.
Ultraviolet radiation (UVR) is probably the most important environmental factor in the induction phase of SLE and especially of LE-specific skin disease. UV light likely leads to self-immunity and loss of tolerance because it causes apoptosis of keratinocytes, which in turn, makes previously cryptic peptides available for immunosurveillance. UVB radiation has been shown to displace autoantigens such as Ro/SS-A and related autoantigens, La/SS-B, and calreticulin, from their normal locations inside epidermal keratinocytes to the cell surface.13 UVB irradiation induces the release of CCL27 (cutaneous T cell-attracting chemokine), which upregulates the expression of chemokines that activate autoreactive T cells and interferon-α (IFN-α), producing dendritic cells (DCs), which likely play a central role in lupus pathogenesis.14,15
A recent, large, case-control study reported that smokers are at a greater risk of developing SLE than are nonsmokers and former smokers. A cross-sectional analysis of a collaborative Web-based database established by Werth and colleagues documented that patients with treatment resistant CLE were much more likely to smoke.16 Several authors have shown that patients with LE-specific skin disease who smoke are less responsive to antimalarial treatment.17–19
Numerous drugs have been implicated in inducing various features of SLE (Table 155-3). The drugs that induce CLE can be linked by their photosensitizing properties. It has been suggested that these drugs cause an increase in keratinocyte apoptosis, exposure of previously intracellular peptides on epidermal cell surfaces, and enhance proinflammatory cytokines such as TNF-α and IFN-α.20,21
There has been much speculation about the role of infectious agents, particularly viruses, in the induction of SLE and CLE. Seroconversion to Epstein-Barr virus (EBV) among patients with SLE is nearly universal, and recent data have demonstrated that patients with SLE have defective control of latent EBV infection that probably stems from altered T-cell responses against EBV.
The Important Role of Innate Immunity in the Pathogenesis of Lupus
![]() DCs have a key role in antigen recognition and stimulation of the immune system. Immature DCs perform a watch-dog like role in all peripheral tissues. They are essential to the maintenance of peripheral tolerance to self-antigens. They constantly sample their environment, capture self-antigens released in normal tissue turnover (apoptosis), and in the absence of inflammation, primarily act as housekeepers, keeping self-antigens in check. In the company of inflammation or microbes, they mature and move to draining lymph nodes, where they present antigen to T cells via MHCs. With appropriate costimulatory molecules, T- and B-cell activation ensues, with subsequent launching of an adaptive immune response.22
DCs have a key role in antigen recognition and stimulation of the immune system. Immature DCs perform a watch-dog like role in all peripheral tissues. They are essential to the maintenance of peripheral tolerance to self-antigens. They constantly sample their environment, capture self-antigens released in normal tissue turnover (apoptosis), and in the absence of inflammation, primarily act as housekeepers, keeping self-antigens in check. In the company of inflammation or microbes, they mature and move to draining lymph nodes, where they present antigen to T cells via MHCs. With appropriate costimulatory molecules, T- and B-cell activation ensues, with subsequent launching of an adaptive immune response.22
![]() DCs develop from precursor monocytes and have two different lineages, myeloid DCs, which then differentiate into immature and mature DCs and plasmacytoid DCs. Plasmacytoid DCs are found in lymphoid organs, such as bone marrow, spleen, tonsils, and lymph nodes, and are noteworthy for producing large amounts of IFN-α in response to many viruses and certain bacteria. They have recently been found to infiltrate LE-specific skin lesions as well. IFN-α causes monocytes to differentiate into myeloid DCs, which are able to capture circulating apoptotic cells. These can then present self-antigens to autoreactive CD4 T cells and also support B-cell proliferation and differentiation, leading to the clinical expression of LE.23
DCs develop from precursor monocytes and have two different lineages, myeloid DCs, which then differentiate into immature and mature DCs and plasmacytoid DCs. Plasmacytoid DCs are found in lymphoid organs, such as bone marrow, spleen, tonsils, and lymph nodes, and are noteworthy for producing large amounts of IFN-α in response to many viruses and certain bacteria. They have recently been found to infiltrate LE-specific skin lesions as well. IFN-α causes monocytes to differentiate into myeloid DCs, which are able to capture circulating apoptotic cells. These can then present self-antigens to autoreactive CD4 T cells and also support B-cell proliferation and differentiation, leading to the clinical expression of LE.23
![]() More recently, several important studies using microarray data, have revealed a genomic “signature” of upregulated IFN-α-inducible genes from peripheral blood mononuclear cells of SLE patients, as well as from lesions of LE-specific skin disease.15 Additionally, it has been shown that IFN-α increases as patients’ disease flares. Recent studies have shown that local production of type I IFNs in CLE induces Th1-biased inflammation via induction of IFN-inducible proteins (MxA) and chemokines (CXCL-9, CXCL-10, and CXCL-11), with recruitment of pathogenic T lymphocytes into the skin.24
More recently, several important studies using microarray data, have revealed a genomic “signature” of upregulated IFN-α-inducible genes from peripheral blood mononuclear cells of SLE patients, as well as from lesions of LE-specific skin disease.15 Additionally, it has been shown that IFN-α increases as patients’ disease flares. Recent studies have shown that local production of type I IFNs in CLE induces Th1-biased inflammation via induction of IFN-inducible proteins (MxA) and chemokines (CXCL-9, CXCL-10, and CXCL-11), with recruitment of pathogenic T lymphocytes into the skin.24
![]() In short, there has been convergence of data from several studies, leading to the suggestion that IFN-α plays a central role in the pathogenesis of lupus.
In short, there has been convergence of data from several studies, leading to the suggestion that IFN-α plays a central role in the pathogenesis of lupus.
![]() Another trigger for IFN-alpha release by DCs appears to be related to a newly described form of neutrophil cell death called “NETosis”. The recent discovery of the chromatin based neutrophil extracellular trap (NET) reveals that NETs are part of the innate immune pathway that immobilize microbes. Antimicrobial peptide LL37 antibody is expressed at high levels in SLE. Along with anti-HNP (heteronucleoprotein) antibodies, anti-LL37 activates neutrophils to release NETs. The production of NETs by these autoantibodies appears to stimulate the production of IFN-alpha by PDCs.24a
Another trigger for IFN-alpha release by DCs appears to be related to a newly described form of neutrophil cell death called “NETosis”. The recent discovery of the chromatin based neutrophil extracellular trap (NET) reveals that NETs are part of the innate immune pathway that immobilize microbes. Antimicrobial peptide LL37 antibody is expressed at high levels in SLE. Along with anti-HNP (heteronucleoprotein) antibodies, anti-LL37 activates neutrophils to release NETs. The production of NETs by these autoantibodies appears to stimulate the production of IFN-alpha by PDCs.24a
![]() It appears that circulating DNA/anti-DNA complexes trigger TLR 7, 8, and 9 signaling, which acts as an amplification loop by inducing proliferation of autoreactive B cells, and IFN-α secretion from DCs. Antimalarials have recently been shown to inhibit TLR signaling, and it may be through this mechanism of action that antimalarials display efficacy in lupus.25
It appears that circulating DNA/anti-DNA complexes trigger TLR 7, 8, and 9 signaling, which acts as an amplification loop by inducing proliferation of autoreactive B cells, and IFN-α secretion from DCs. Antimalarials have recently been shown to inhibit TLR signaling, and it may be through this mechanism of action that antimalarials display efficacy in lupus.25
![]() TNF is likely involved in the pathogenesis of cutaneous lupus. TNF induces apoptosis through the FAS-associated death domain. TNF has been shown to be elucidated by keratinocytes exposed to UVR. TNF is produced in large amounts by epidermal keratinocytes in patients with SCLE.26 However, there have also been numerous reports of the development or worsening of cutaneous lupus while on anti-TNF therapy; and the development of dsDNA antibodies and lupus-like syndromes is well described with drugs such as etanercept and infliximab. This is likely due to the upregulation of IFN by TNF inhibition, disrupting the homeostasis of these two proinflammatory cytokines.27
TNF is likely involved in the pathogenesis of cutaneous lupus. TNF induces apoptosis through the FAS-associated death domain. TNF has been shown to be elucidated by keratinocytes exposed to UVR. TNF is produced in large amounts by epidermal keratinocytes in patients with SCLE.26 However, there have also been numerous reports of the development or worsening of cutaneous lupus while on anti-TNF therapy; and the development of dsDNA antibodies and lupus-like syndromes is well described with drugs such as etanercept and infliximab. This is likely due to the upregulation of IFN by TNF inhibition, disrupting the homeostasis of these two proinflammatory cytokines.27
![]() T cells play a key role in both the induction and expansion phases in the development of SLE. T cells are involved in the development of both central and peripheral tolerance. Self-antigens are presented by DCs to autoreactive T cells. Binding of the surface signaling molecules, such as the T-cell receptor, to their ligands leads to T-cell activation. In SLE, and in CLE, T cells display markers of increased activation, increased numbers of DR+ antigens.28 T cells also provide cognate help to autoreactive B cells, leading to production of autoantibodies.
T cells play a key role in both the induction and expansion phases in the development of SLE. T cells are involved in the development of both central and peripheral tolerance. Self-antigens are presented by DCs to autoreactive T cells. Binding of the surface signaling molecules, such as the T-cell receptor, to their ligands leads to T-cell activation. In SLE, and in CLE, T cells display markers of increased activation, increased numbers of DR+ antigens.28 T cells also provide cognate help to autoreactive B cells, leading to production of autoantibodies.
![]() B cells are involved in the expansion phase of LE pathogenesis, in that they can present antigen to autoreactive T cells and further amplify T-cell activation. The production by B cells of autoantibodies against nuclear antigens is the hallmark of SLE.29 Several of these autoantibodies are thought to be directly pathogenic, including dsDNA and Ro/SS-A antibodies. Such autoantibodies likely play a direct role in the injury phase of LE. They form immune complexes, which may cause tissue damage by means that include direct cell death, cellular activation, opsonization, and the blocking of target molecule function. B cells may not be as important in CLE as they are in other manifestations of the disease, as supported by the suggestion that anti-B-cell therapies such as rituximab are less effective for CLE.
B cells are involved in the expansion phase of LE pathogenesis, in that they can present antigen to autoreactive T cells and further amplify T-cell activation. The production by B cells of autoantibodies against nuclear antigens is the hallmark of SLE.29 Several of these autoantibodies are thought to be directly pathogenic, including dsDNA and Ro/SS-A antibodies. Such autoantibodies likely play a direct role in the injury phase of LE. They form immune complexes, which may cause tissue damage by means that include direct cell death, cellular activation, opsonization, and the blocking of target molecule function. B cells may not be as important in CLE as they are in other manifestations of the disease, as supported by the suggestion that anti-B-cell therapies such as rituximab are less effective for CLE.
![]() Clearly, there are several key constituents involved in the pathogenesis of LE. The sheer number of potential players in disease development is one the main reasons for the highly variable expression of the disease. Recent data uncovering convergence points (i.e., IFN-α) in the pathogenetic expression of disease have already led to early development of new drugs for LE. Additionally, the complexity of the pathogenesis of LE suggests that multiple pharmacotherapeutics (a layering of drugs with different pathomechanisms) may be required to treat patients with recalcitrant disease.
Clearly, there are several key constituents involved in the pathogenesis of LE. The sheer number of potential players in disease development is one the main reasons for the highly variable expression of the disease. Recent data uncovering convergence points (i.e., IFN-α) in the pathogenetic expression of disease have already led to early development of new drugs for LE. Additionally, the complexity of the pathogenesis of LE suggests that multiple pharmacotherapeutics (a layering of drugs with different pathomechanisms) may be required to treat patients with recalcitrant disease.
Clinical Findings
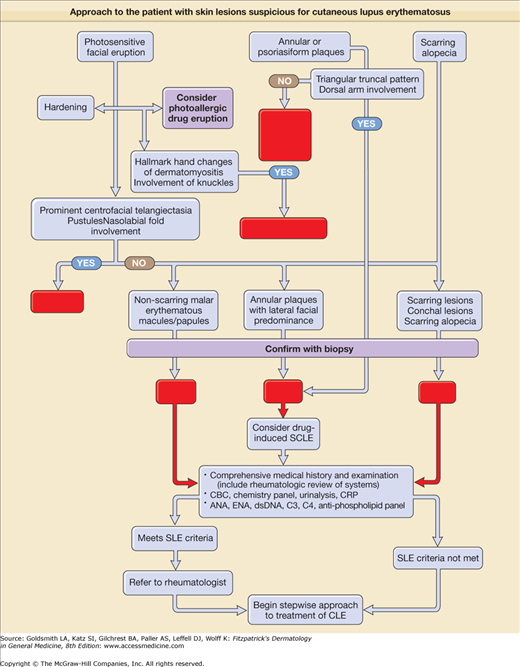
eFigure 155-0.2
Approach to the patient with skin lesions suspicious for cutaneous lupus erythematosus. ACLE = acute cutaneous lupus erythematosus; ANA = antinuclear antibody; CBC = complete blood cell count; CLE = cutaneous lupus erythematosus; CRP = C-reactive protein; DLE = discoid lupus erythematosus; dsDNA = doubled-stranded DNA; EAC = erythema annulare centrifugum; ENA = extractable nuclear antibody; GA = granuloma annulare; SCLE = subacute cutaneous lupus erythematosus; SLE = systemic lupus erythematosus.
eTable 155-3.1 compares the key clinical, histopathologic, and laboratory features of patients presenting with ACLE, SCLE, and CCLE (classic DLE) skin lesions. It is important to distinguish among the subtypes of LE-specific skin disease, because the type of skin involvement in LE can reflect the underlying pattern of SLE activity. In fact, the designations acute, subacute, and chronic, in regard to CLE, refer to the pace and severity of any associated SLE and are not necessarily related to how long individual lesions have been present. For example, ACLE almost always occurs in the setting of acutely flaring SLE, whereas CCLE often occurs in the absence of SLE or in the presence of mild smoldering SLE. SCLE occupies an intermediate position in this clinical spectrum. Subclassification, although important for assigning risk, is sometimes difficult, as it is not uncommon to see more than one subtype of LE-specific skin disease in the same patient, especially in patients with SLE.
Disease Features | ACLE | SCLE | Classic DLE |
|---|---|---|---|
Clinical features of skin lesions | |||
Induration Dermal atrophy Pigment changes Follicular plugging Hyperkeratosis | 0 0 + 0 + | 0 0 ++ 0 ++ | +++ +++ +++ +++ +++ |
Histopathology | |||
Thickened basement membrane Lichenoid infiltrate Periappendageal inflammation | 0 + 0 | + ++ + | +++ +++ +++ |
Lupus band | |||
Lesional Nonlesional | ++ ++ | ++ + | +++ 0 |
Antinuclear antibodies | +++ | ++ | + |
Ro/SS-A antibodies | |||
By immunodiffusion By ELISA | + ++ | +++ +++ | 0 + |
Antidouble-stranded DNA antibodies | +++ | + | 0 |
Hypocomplementemia | +++ | + | + |
Risk for developing systemic lupus erythematosus | +++ | ++ | + |
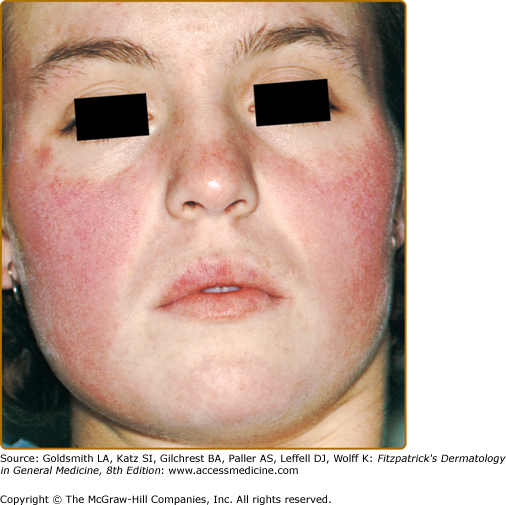
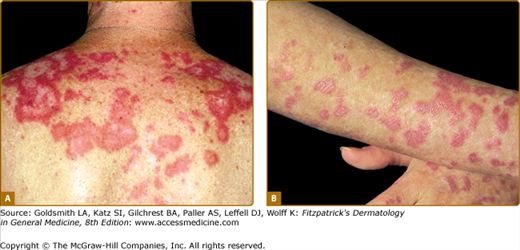
Although ACLE localized to the face is the usual pattern of presentation, ACLE can assume a generalized distribution. Localized ACLE has commonly been referred to as the classic butterfly rash or malar rash of SLE (Fig. 155-1). In localized ACLE, confluent symmetric erythema and edema are centered over the malar eminences and bridges over the nose (unilateral involvement with ACLE has been described). The nasolabial folds are characteristically spared. The forehead, chin, and V area of the neck can be involved, and severe facial swelling may occur. Occasionally, ACLE begins as small macules and/or papules on the face that later may become confluent and hyperkeratotic. Generalized ACLE presents as a widespread morbilliform or exanthematous eruption often focused over the extensor aspects of the arms and hands and characteristically sparing the knuckles (Fig. 155-2A). Although perivascular nail fold erythema and telangiectasia can occur (see Fig. 155-2B), they are considerably more common and occur in more exaggerated forms in dermatomyositis (see Fig. 157-5). Generalized ACLE has been indiscriminately referred to as the maculopapular rash of SLE, photosensitive lupus dermatitis, and SLE rash. An extremely acute form of ACLE is rarely seen that can simulate toxic epidermal necrolysis (TEN). This form of LE-specific vesiculobullous disease results from widespread apoptosis of epidermal keratinocytes, and eventuates in areas of full-thickness epidermal skin necrosis, which is subsequently denuded. It can be differentiated between true TEN because it occurs on predominantly sun-exposed skin and has a more insidious onset.30 The mucosa may or may not be involved, as in TEN.
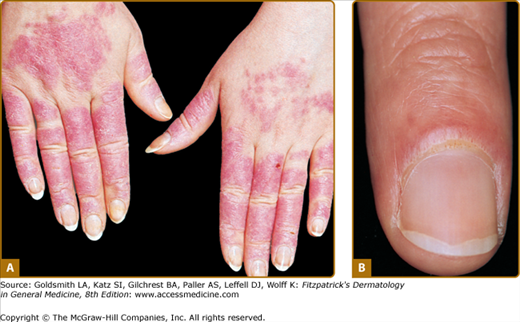
Figure 155-2
Generalized acute cutaneous lupus erythematosus. A. Well-demarcated patches of erythema with fine overlying scale on the dorsal aspect of the hands, fingers, and periungual areas. Note the characteristic sparing of the knuckles, which are preferentially involved in dermatomyositis. B. Closeup view of periungual erythema and grossly visible telangiectasia. Although these lesions can be seen in lupus erythematosus, they are more typical of dermatomyositis.
ACLE is typically precipitated or exacerbated by exposure to UV light. This form of CLE can be quite ephemeral, lasting only hours, days, or weeks; however, some patients experience more prolonged periods of activity. Postinflammatory pigmentary change is most prominent in patients with heavily pigmented skin. Scarring does not occur in ACLE unless the process is complicated by secondary bacterial infection.
A disease presentation dominated by SCLE lesions marks the presence of a distinct subset of LE having characteristic clinical, serologic, and genetic features. Although a finding of circulating autoantibodies to the Ro/SS-A ribonucleoprotein particle strongly supports a diagnosis of SCLE, the presence of this autoantibody specificity is not required to make a diagnosis of SCLE.
SCLE initially presents as erythematous macules and/or papules that evolve into hyperkeratotic papulosquamous or annular/polycyclic plaques (Fig. 155-3). Whereas most patients have either annular or papulosquamous SCLE, a few develop elements of both morphologic varieties. SCLE lesions are characteristically photosensitive and occur in predominantly sun-exposed areas (i.e., upper back, shoulders, extensor aspects of the arms, V area of the neck, and, less commonly, the face). SCLE lesions typically heal without scarring but can resolve with long-lasting, if not permanent, vitiligo-like leukoderma, and telangiectasias.
Several variants of SCLE have been described. On occasion, SCLE lesions present initially with an appearance of erythema multiforme. Such cases are similar to Rowell syndrome (erythema multiforme-like lesions occurring in patients with SLE in the presence of La/SS-B autoantibodies). As a result of intense injury to epidermal basal cells, the active edge of an annular SCLE lesion occasionally undergoes a vesiculobullous change that can subsequently produce a strikingly crusted appearance. Such lesions can mimic Stevens-Johnson syndrome/TEN. Pathogenesis is similar to that described above for TEN-like ACLE. Rarely, SCLE presents with exfoliative erythroderma or displays a curious acral distribution of annular lesions. Pityriasiform and exanthematous variants of SCLE have been reported. The skin lesions of neonatal LE (transient, photosensitive, nonscarring LE-specific skin lesions in neonates who have received IgG anti-Ro/SS-A, and, occasionally, other autoantibody specificities transplacentally) share many features with SCLE.
Unlike ACLE skin lesions, SCLE lesions tend to be less transient than ACLE lesions and heal with more pigmentary change. They are also less edematous and more hyperkeratotic than ACLE lesions. SCLE more commonly involves the neck, shoulders, upper extremities, and trunk, whereas ACLE more commonly affects the malar areas of the face. When the face is involved with SCLE, it is most often the lateral face, with sparing of the central, malar regions. In comparison to SCLE lesions, DLE lesions are generally associated with a greater degree of hyper- and hypopigmentation, atrophic dermal scarring, follicular plugging, and adherent scale. A consistent clinical difference is that DLE lesions are characteristically indurated, whereas SCLE lesions are not; this difference reflects the greater depth of inflammation seen histopathologically in DLE lesions.
Approximately one-half of patients with SCLE meet the ACR’s revised criteria for the classification of SLE. However, manifestations of severe SLE, such as nephritis, central nervous system disease, and systemic vasculitis, develop in only 10%–15% of patients with SCLE. It has been suggested that the papulosquamous type of SCLE, leu-kopenia, high titer of antinuclear antibody (ANA) (>1:640), and anti-dsDNA antibodies are risk factors for the development of SLE in a patient presenting with SCLE lesions.
SCLE can overlap with other autoimmune diseases, including Sjögren’s syndrome, rheumatoid arthritis, and Hashimoto’s thyroiditis. Other disorders that have been anecdotally related to SCLE are Sweet syndrome, porphyria cutanea tarda, gluten-sensitive enteropathy, and Crohn’s disease. There has also been the suggestion that SCLE can be associated with internal malignancy (breast, lung, gastric, uterine, hepatocellular, and laryngeal carcinomas as well as with Hodgkin lymphoma).31
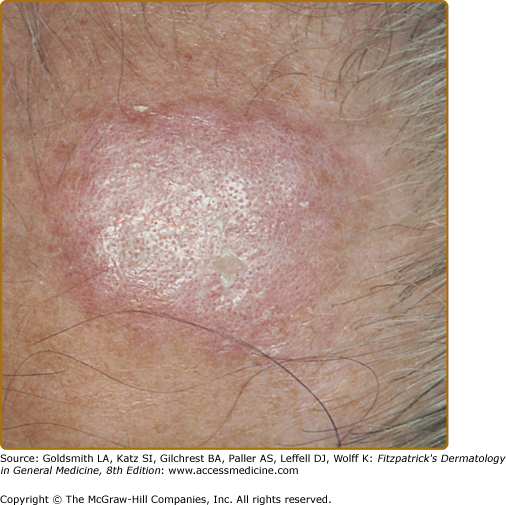
Classic DLE lesions, the most common form of CCLE, begin as red-purple macules, papules, or small plaques and rapidly develop a hyperkeratotic surface. Early classic DLE lesions typically evolve into sharply demarcated, coin-shaped (i.e., discoid) erythematous plaques covered by a prominent, adherent scale that extends into the orifices of dilated hair follicles (Fig. 155-4).
Figure 155-4
Classic discoid lupus erythematosus. Typical early erythematous plaque on the forehead demonstrating hyperkeratosis and accentuation of follicle orifices in a 60-year-old man with a 25-year history of cutaneous lupus erythematosus. The lesion had been present for 3 months; no dermal atrophy was present at this stage.
DLE lesions typically expand with erythema and hyperpigmentation at the periphery, leaving hallmark atrophic central scarring, telangiectasia, and hypopigmentation (Fig. 155-5). DLE lesions at this stage can merge to form large, confluent, disfiguring plaques. DLE in persons of certain ethnic backgrounds, such as Asian Indians, can present clinically as isolated areas of macular hyperpigmentation. When present on hair-bearing skin (scalp, eyelid margins, and eyebrows), DLE causes scarring alopecia, which can lead to disfigurement and markedly impact quality of life. Follicular involvement in DLE is a prominent feature. Keratotic plugs accumulate in dilated follicles that soon become devoid of hair. When the adherent scale is lifted from more advanced lesions, keratotic spikes similar in appearance to carpet tacks can be seen to project from the undersurface of the scale (i.e., the “carpet tack” sign). DLE lesions can be difficult to diagnose in Caucasian patients because the characteristic peripheral hyperpigmentation is often absent. Such lesions are often confused with actinic keratoses, squamous cell carcinoma, or acne.
Figure 155-5
Classic discoid lupus erythematosus. Sharply demarcated, round-to-ovoid slightly indurated, erythematous plaques on the neck and face. Most plaques show a mild degree of hyperkeratosis, and some show dermal atrophy. Noninflamed areas of hypopigmentation and scarring mark the sites of prior lesions that have resolved.
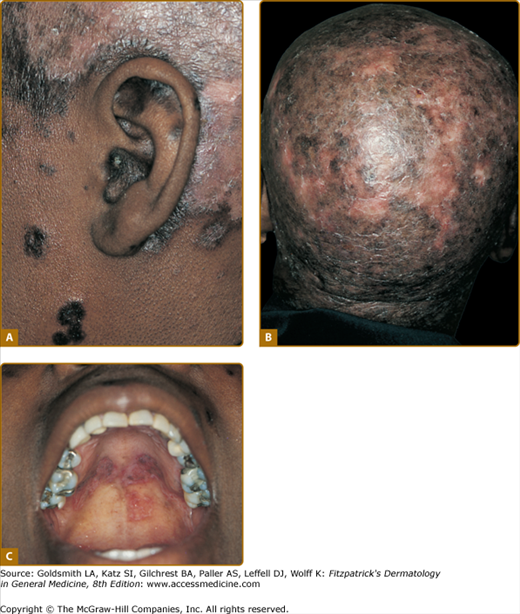
DLE lesions are most frequently encountered on the face, scalp, ears, V area of the neck, and extensor aspects of the arms. Any area of the face, including the eyebrows, eyelids, nose, and lips, can be affected. A symmetric, hyperkeratotic, butterfly-shaped DLE plaque is occasionally found over the malar areas of the face and bridge of the nose. Such lesions should not be confused with the more transient, edematous, minimally scaling ACLE erythema reactions that occur in the same areas. Facial DLE, like ACLE and SCLE, usually spares the nasolabial folds. It may be difficult to distinguish early lesions of malar DLE from ACLE, but induration and recalcitrance to topical steroids/calcineurin inhibitors favors the former diagnosis. When DLE lesions occur periorally, they resolve with a striking acneiform pattern of pitted scarring. DLE characteristically affects the external ear, including the outer portion of the external auditory canal (Fig. 155-6A). Such lesions often present initially as dilated, hyperpigmented follicles. The scalp is involved in 60% of patients with DLE; irreversible, scarring alopecia resulting from such involvement has been reported in one-third of patients (see Fig. 155-6B). The irreversible, scarring alopecia resulting from DLE differs from the reversible, nonscarring alopecia that patients with SLE often develop during periods of systemic disease activity. This type of hair loss, so-called lupus hair, may be telogen effluvium occurring as the result of flaring systemic disease.
Figure 155-6
Classic discoid lupus erythematosus (DLE) and mucosal DLE in a 45-year-old African-American woman with a 20-year history of untreated cutaneous lupus erythematosus. A. Characteristic involvement of the ear shows lesions with atrophy and postinflammatory hyperpigmentation as well as inflammatory red plaques on the scalp with postinflammatory hypopigmentation. B. Confluent lesions on the scalp have resulted in extensive scarring alopecia. C. Plaques of DLE on the palatal mucosa showing morphologic features similar to those of cutaneous lesions.
Localized DLE lesions occur only on the head or neck, whereas generalized DLE lesions occur both above and below the neck. Generalized DLE is more commonly associated with underlying SLE and is often more recalcitrant to standard therapy, frequently requiring layering of antimalarial and immunosuppressive medications. DLE lesions below the neck most commonly occur on the extensor aspects of the arms, forearms, and hands, although they can occur at virtually any site on the body. The palms and soles can be the sites of painful, and at times disabling, erosive DLE lesions. On occasion, small DLE lesions occurring only around follicular orifices appear at the elbow and elsewhere (follicular DLE). We have observed that elbow/extensor arm lesions seem to cooccur with acral finger lesions of DLE, and that patients with this combination of findings more frequently have active systemic disease. DLE activity can localize to the nail unit. The nail can be impacted by other forms of CLE as well as SLE, producing nail fold erythema and telangiectasia, red lunulae, clubbing, paronychia, pitting, leukonychia striata, and onycholysis.
DLE lesions can be potentiated by sunlight exposure but to a lesser extent than ACLE and SCLE lesions. DLE, as well as other forms of LE skin disease activity, can be precipitated by any form of cutaneous trauma (i.e., the Koebner phenomenon or isomorphic effect).
The relationship between classic DLE and SLE has been the subject of much debate.32 The following summary points can be made: (1) 5% of patients presenting with classic DLE lesions subsequently develop unequivocal evidence of SLE and (2) patients with generalized DLE (i.e., lesions both above and below the neck) have somewhat higher rates of immunologic abnormalities, a higher risk for progressing to SLE, and a higher risk for developing more severe manifestations of SLE than patients with localized DLE.
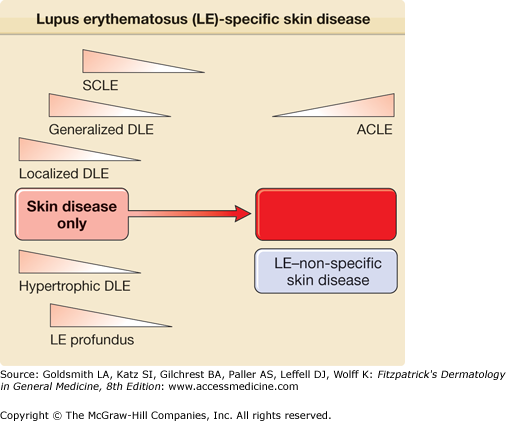
Roughly one-fourth of patients with SLE develop DLE lesions at some point in the course of their disease, and such patients tend to have less severe forms of SLE. Figure 155-7 illustrates the relative risks for systemic disease activity that are associated with the clinical varieties of LE-specific skin disease.
Figure 155-7
Relative risk for coexistence of or progression to systemic lupus erythematosus in patients presenting with various forms of lupus erythematosus (LE)-specific skin disease. The spectrum of LE is depicted as a disease continuum ranging from skin disease alone to life-threatening systemic disease. ACLE = acute cutaneous lupus erythematosus; DLE = discoid lupus erythematosus; SCLE = subacute cutaneous lupus erythematosus.
Aside from Classic DLE, there are several other less common variants of CCLE, which are subclasssified as such because of their overlapping histologies and tendency to occur in a low frequency in association with underlying SLE.
Hypertrophic DLE, also referred to as hyperkeratotic or verrucous DLE, is a rare variant of CCLE in which the hyperkeratosis normally found in classic DLE lesions is greatly exaggerated. The extensor aspects of the arms, the upper back, and the face are the areas most frequently affected. Overlapping features of hypertrophic LE and lichen planus have been described under the rubric lupus planus. The entity lupus erythematosus hypertrophicus et profundus appears to represent a rare form of hypertrophic DLE, affecting the face with the additional features of violaceous/dull red, indurated, rolled borders and striking central, crateriform atrophy. The name for this clinical entity is ambiguous because LE panniculitis is not characteristic of its histopathology. Patients with hypertrophic DLE probably do not have a greater risk for developing SLE than do patients with classic DLE lesions.
Mucosal DLE occurs in approximately 25% of patients with CCLE. The oral mucosa is most frequently affected; however, nasal, conjunctival, and genital mucosal surfaces can be targeted. In the mouth, the buccal mucosal surfaces are most commonly involved, with the palate (see Fig. 155-6C), alveolar processes, and tongue being sites of less frequent involvement. Lesions begin as painless, erythematous patches that evolve to chronic plaques that can be confused with lichen planus. Chronic buccal mucosal plaques are sharply marginated and have irregularly scalloped, white borders with radiating white striae and telangiectasia. The surfaces of these plaques overlying the palatal mucosa often have a honeycomb appearance. Central depression often occurs in older lesions, and painful ulceration can develop. Rarely, oral mucosal DLE lesions can degenerate into squamous cell carcinoma, similar to longstanding cutaneous DLE lesions. Any degree of nodular asymmetry within a mucosal DLE lesion should be evaluated for the possibility of malignant degeneration. Chronic DLE plaques also appear on the vermilion border of the lips. At times, DLE involvement of the lips can present as a diffuse cheilitis, especially on the more sun-exposed lower lip.
DLE lesions may present on the nasal, conjunctival, and anogenital mucosa. Perforation of the nasal septum is more often associated with SLE than DLE. Conjunctival DLE lesions affect the lower lid more often than the upper lid. Lesions begin as focal areas of nondescript inflammation most commonly affecting the palpebral conjunctivae or the lid margin. Scarring becomes evident as lesions mature, and the permanent loss of eyelashes and ectropion can develop, producing considerable disability.
LE profundus/LE panniculitis (Kaposi-Irgang disease) is a rare form of CCLE typified by inflammatory lesions in the lower dermis and subcutaneous tissue. Approximately 70% of patients with this type of CCLE also have typical DLE lesions, often overlying the panniculitis lesions. Some have used the term LE profundus to designate those patients who have both LE panniculitis and DLE lesions, and LE panniculitis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree