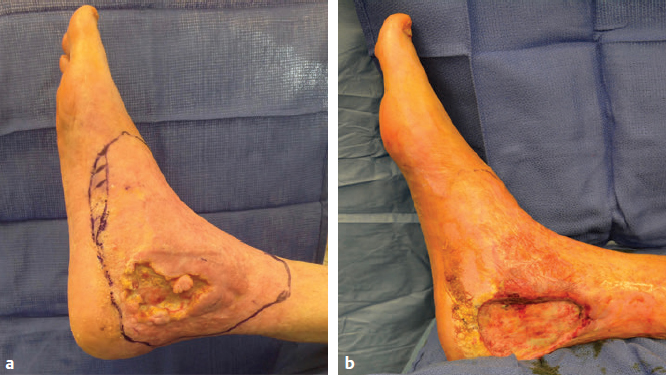
CHAPTER Lower extremity reconstruction is a multidisciplinary field requiring coordination of care by multiple specialties including trauma surgery, orthopedic surgery, plastic surgery, vascular surgery, podiatry, radiology, physical therapy, occupational therapy, prosthetics, physical medicine and rehabilitation, psychiatry, and pain management. Plastic surgeons are commonly asked to provide advanced expertise in the management of problematic lower extremity wounds secondary to trauma, vascular disease, diabetic ulcers, infections, or oncologic resection. The primary goals of lower limb reconstruction are to preserve function and limb length, achieve stable bony fixation, and provide du rable soft tissue reconstruction to withstand load-bearing and shearing forces and allow pain-free ambulation. This chapter reviews reconstructive considerations of the lower extremity with special emphasis on perioperative planning, avoidance of complications, and management of unfavorable results. Summary Box Complications in Lower Extremity Reconstruction • Inadequate preoperative planning • Improper flap design • Vascular problems • Deep venous thrombosis or pulmonary embolism • Recurrent ulceration related to a biomechanical problem with gait • Infection • Osteomyelitis • Partial flap loss • Complete flap loss • Bulky flap • Noncompliance with postoperative protocol • Reconstruction of nonfunctional limb Proper patient evaluation includes several steps: • A comprehensive past medical and surgical history • Assessment of comorbidities: • Assessment of the patient’s expectations, motivation for rehabilitation, psychological status, job requirements, and level of social support • A history of chemotherapy or radiation therapy, which are equally pertinent and can adversely affect wound healing and reconstructive outcome • Assessment and optimization of the patient’s nutritional status • Preoperative imaging such as computed tomography angiography (CTA), magnetic resonance angiography (MRA), or angiography should be assessed For patients with underlying renal disease or a contrast allergy, it is important to note that a properly executed angiogram uses far less intravenous contrast dye than does CTA. Other indicated vascular studies such as duplex ultrasound to assess for venous insufficiency or underlying DVT should be obtained preoperatively to assess venous outflow. Effective communication with other involved services such as anesthesia, trauma surgery, orthopedic surgery, internal medicine, and physical therapy is critical. In patients with severe illnesses and comorbidities, amputation may be a better option than aggressive attempts at limb salvage, which can lead to costly medical complications. Accurate wound assessment and adequate débridement are integral to lower extremity reconstruction. Complex reconstruction surgery techniques are used to cover bone, tendon, hardware, or other vital structures. Ensuring that the wound bed has been adequately débrided, is clean, and shows signs of granulation tissue is essential to a successful, durable reconstruction. Deep postdébridement tissue cultures are helpful to determine whether the wound is ready for closure and will prevent late osteomyelitis or late infection.1 Although initial studies in the trauma literature advocated earlier coverage within 72 hours,2 such an approach is not always necessary and may not always yield optimal results.3,4 Additionally, the use of negative-pressure wound therapy (NPWT) has made a positive impact on wound management, and delayed coverage of the wound has not resulted in increased flap failure rates.3,5 Although some wounds can be covered early, other wounds with greater soft tissue injury may require 7 to 10 days to fully demarcate and undergo adequate débridement. In such scenarios, serial débridement every 48 to 72 hours and appropriate NPWT management are necessary before definitive coverage. Newer technology with NPWT and instillation of fluid has been shown to decrease length of stay and timing of closure in lower extremity reconstruction.6 Expeditious coverage of exposed hardware, major vessels, nerves, joint surfaces, tendons, and bone is attempted whenever possible to avoid later complications. Intraoperative execution of the reconstructive plan by following meticulous surgical technique is paramount. The surgeon and anesthesia team must pay close attention to maintenance of patient normothermia, adequate padding of pressure points, proper patient positioning for the intended flap elevation and inset dressings, effective DVT prophylaxis, and prospective hemostasis. Examination of potential flap donor sites for any signs of trauma or previous surgical intervention that may compromise flap viability is important. For foot and lower leg reconstruction, the ideal flap donor site should be thin enough to later fit into a shoe. If there was soft tissue infection or osteomyelitis, a culture-specific antibiotic regimen is paramount to the immediate and long-term success of any extremity reconstruction. Surgical options for lower extremity reconstruction are local or free tissue transfers. Local flaps include local pedicled fasciocutaneous flaps, such as perforator-propeller flaps that have been recently popularized as options in lieu of free flaps.7 These flaps are technically demanding and require a learning curve. Careful flap selection is key in avoiding complications. Patient age older than 60 years, diabetes, and peripheral vascular disease are significant risk factors for perforator–pedicled propeller flap complications in the lower extremity.8 For lower extremity free tissue transfer, proper flap selection, flap design, careful elevation and recipient vessel selection are prerequisites for success. Free flap failure is generally higher in lower extremity reconstruction compared with breast and head and neck reconstruction.9 The most important consideration to avoid free flap loss is early recognition of flap compromise, which can be more difficult to assess in muscle flaps versus skin flaps (Fig. 58.1). Flap loss rates of up to 28% have been reported. Vascular complications remain the primary reason for free flap loss. As stated previously, preoperative assessment of arterial and venous flow should be confirmed by physical examination and duplex ultrasound when venous insufficiency or edema is suspected. Preoperative angiogram, CTA, or MRA are indicated for virtually every patient. Fig. 58.1 Early signs of venous congestion in a free muscle flap. (a) Preoperative view of a large posterior leg and heel defect. (b) Immediate postoperative view after free vastus lateralis muscle flap and skin graft. The muscle is soft and pink under the skin graft. (c) Approximately 14 hours postoperatively, the wound shows early signs of venous congestion with purplish muscle, edema, and a darker-colored skin graft. Any change from the immediate postoperative examination should warrant taking the patient back to the operating room. The recipient vessels should be explored and assessed for pulsatile flow and suitability for microvascular anastomosis before flap elevation. Because of the nature of the compartmental trauma of the lower extremity and the resulting thrombogenic zone of injury, selecting vessels proximal to the zone of injury is preferred whenever possible. However, this may not always be feasible. Meticulous microsurgical technique distal to the zone of injury has been safely performed, with reported success rates up to 97% in some centers.10 Advantages of using recipient vessels distally include easier access, technically easier anastomosis, shorter flap pedicle requirement, no need for vein graft or tunneling the pedicle, and avoiding crossing the knee joint. Using the distal recipient vessels is possible, but inset of the flap is more complex, because the venous outflow must take a 180-degree turn from the flap to the recipient venous outflow. It is important to have adequate length to make sure the pedicle is not kinked. In general, end-to-side arterial anastomosis has equivalent free flap success rates to end-to-end anastomosis and should be considered in most extremity reconstruction to preserve distal arterial supply.11 Most importantly, end-to-side anastomosis avoids issues with size mismatch. In one large series, an end-to-side arterial anastomosis technique was performed in 50% of patients with a single vessel run off without an increased incidence of foot ischemia or flap failure rates when compared with the end-to-end arterial anastomosis technique.12 Similarly, in another large oncologic series, end-to-end arterial anastomosis had equivalent flap outcomes to end-to-side anastomosis. However, end-to-side venous anastomosis was strongly associated with unplanned reoperation and total flap loss.13 After microvascular anastomosis, if flap perfusion is questionable, the microvascular anastomosis must be assessed before leaving the operating room, where most vascular compromise begins.14 In general, venous insufficiency is more common than arterial thrombotic complications in free flap reconstruction of the lower extremity.15 In a review of 170 free flaps, when the deep vein system was used in microvascular anastomosis, a higher risk for DVT measured by the Caprini risk assessment model was shown to be predictive of flap venous insufficiency after microsurgical lower extremity reconstruction.16 In our experience, performing two venous anastomoses is preferred whenever possible. It is important to assess the back bleeding of the venous system before performing the microsurgical anastomosis to ensure the pressure in the venous system is not too high. Our preference is to perform microsurgical anastomosis to the deep venous system, because usually the venae comitantes are adjacent to the artery and easily accessible. A second venous anastomosis to the superficial system is recommended, when feasible. Adding a second venous anastomoses has been shown to decrease the incidence of flap failure rate by 36% and venous thrombosis rate by 34% compared with one venous anastomoses.17 One of the most important considerations when performing lower extremity free tissue transfer is assessing the final wound closure for tightness, potential pedicle compression, and kinking, which can lead to thrombosis. The flap design should always be larger than the defect and take in consideration the coverage of the pedicle outside the zone of injury to prevent tension and kinking. Use of the implantable Cook Doppler may be helpful during inset to ensure that the closure is not compressing the venous outflow. It is always better to leave portions of the defect open if the closure is too tight and compressing the pedicle. With the advent of imaging systems such as SPY (Novadaq), cutaneous tissue perfusion can easily be assessed; if questionable, the time to intervene is in the operating room. The application of proper dressings and splints is important at the end of the procedure; dressings should be loose with very light compression and splint immobilization to prevent motion but not compress the microvascular anastomosis or flap itself. Fig. 58.2 Prolonged dangling leading to signs of venous congestion. (a) Free anterolateral thigh flap for coverage of a tibial fracture. (b) Dangling began on postoperative day 3 without compression. Venous congestion is visible. (c) After stopping the dangling protocol and restarting a couple of days later, the flap is well healed without adverse sequelae. Positional tension and edema can also occur postoperatively during dangling or mobility. Institution of a dangling protocol is important to allow the venous outflow to equilibrate and allow for dependent high flow.18,19 In general patients maintain leg elevation for 5 to 7 days before starting a dangling protocol. Pressure off-loading, limb elevation, and continuous flap monitoring in the early postoperative period and during dangling protocol are important considerations (Fig. 58.2). Hematomas and fluid collections should be drained in a timely manner to avoid flap compromise. Trauma is a common presentation requiring lower extremity reconstruction. Trauma victims are generally young and healthy individuals with an active lifestyle. Motor vehicle accidents, motorcycle accidents, industrial trauma, and military trauma account for most of these injuries. Some of these injuries are not only limb-threatening, but also life-threatening. Limb salvage and reconstruction should always be considered within the context of the patient’s overall physiologic state. At initial presentation, stabilization of the patient and immediate attention to any life-threatening injuries is undertaken according to advanced cardiac life support (ACLS) and advanced trauma life support (ATLS) guidelines. Focused clinical examinations to diagnose and assess vascular injury, fracture displacement, tissue perfusion, limb sensibility, extent of soft tissue compromise, and bony injury is a must. CTA has become the standard of care in assessing lower extremity vascular injury in most institutions. High-resolution vascular images can be obtained quickly and cost-effectively without the need to assemble a specialized team for catheter-directed angiography or its associated morbidity. Fractures are evaluated simultaneously. In our experience with CTA in preoperative planning for lower extremity trauma and free tissue transfer, up to 64% of patients with findings of traumatic injury on CT had a documented normal peripheral vascular examination.20 Signs of vascular damage include filling arterial defects, extravasation of contrast, vessel narrowing, or early venous opacification. CTA is also valuable in assessing variable vascular anatomy and in preoperative planning for recipient vessel selection for free tissue transfer.21 Specialized monitoring systems such as measurement of intracompartmental pressures and the performance of fasciotomies in a timely fashion are often required. Careful examination under anesthesia and serial assessment of limb perfusion and wound débridement can better delineate the damaged structures and the final defect to be reconstructed. In hemodynamically unstable patients, definitive reconstruction should be delayed and the wound should be temporized. Fracture reduction, wound débridement, and wound vacuum-assisted closure may be required. Table 58.1 Mangled Extremity Severity Score (MESS) scoring system
58
Lower Extremity Reconstruction
Avoiding Unfavorable Results and Complications in Lower Extremity Reconstruction
 Poor medical optimization
Poor medical optimization
 Improper wound assessment
Improper wound assessment
 Underestimation of zone of injury
Underestimation of zone of injury
 Improper assessment of vessel suitability
Improper assessment of vessel suitability
 Poor understanding of patient’s preoperative function
Poor understanding of patient’s preoperative function
 Missed diagnosis of underlying malignancy, radiation, or indolent infection
Missed diagnosis of underlying malignancy, radiation, or indolent infection
 Venous insufficiency
Venous insufficiency
 Arterial thrombosis
Arterial thrombosis
Proper Preoperative Planning and Patient Selection to Avoid Complications
Patient Selection
 Diabetes
Diabetes
 Smoking
Smoking
 Peripheral vascular disease
Peripheral vascular disease
 Prior lower extremity trauma
Prior lower extremity trauma
 Cardiovascular disease
Cardiovascular disease
 Hypercoagulability
Hypercoagulability
 Deep venous thrombosis (DVT)
Deep venous thrombosis (DVT)
 Lymphedema
Lymphedema
 Obesity
Obesity
 Steroid use
Steroid use
 Drug dependency
Drug dependency
Timing of Closure
Overview of Surgical Technique
Trauma
Variable | Points |
Skeletal/soft tissue injury | |
Low energy (stab; simple fracture; civilian gunshot wound) | 1 |
Medium energy (open or multiple fracture, dislocation) | 2 |
High energy (close-range shotgun or military gunshot wound, crush injury) | 3 |
Very high energy (above criteria plus gross contamination, soft tissue avulsion) | 4 |
Limb ischemia | |
Pulse reduced or absent but normal perfusion | 1* |
Pulseless, paresthesias, diminished capillary refill | 2* |
Cool, paralyzed, insensate, numb | 3* |
Shock | |
Systolic blood pressure always > 90 mm Hg | 0 |
Transient hypotension | 1 |
Persistent hypotension | 2 |
Age | |
Younger than 30 years of age | 0 |
Between 30 and 50 years of age | 1 |
Older than 50 years of age | 2 |
Amputation versus Salvage
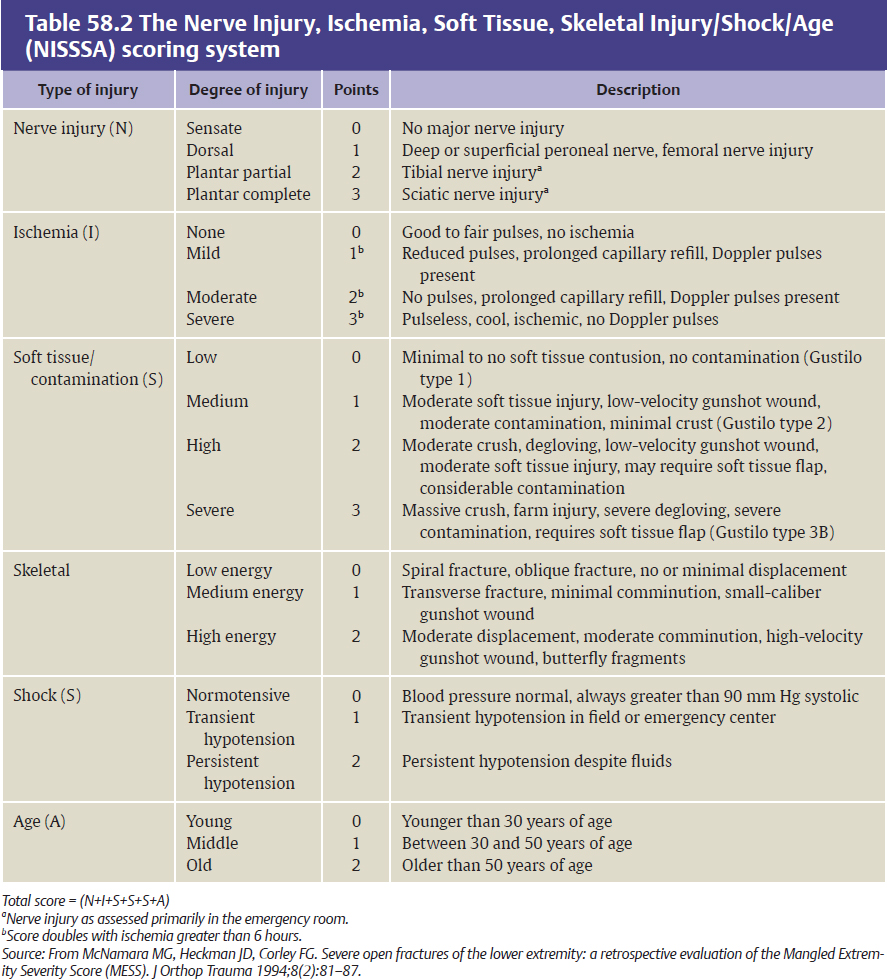
The plastic surgeon plays an integral role along with the orthopedic surgeon in providing insight and expertise on reconstructive options for lower extremity wounds. Several scoring systems have been proposed to help surgeons make the decision to salvage or amputate the injured limb. The Mangled Extremity Severity Score (MESS) and Nerve Injury/Ischemia/Soft tissue/Skeletal Injury/Shock/Age (NISSSA) score are useful scoring systems that help the surgical team make decisions about limb salvage versus amputation.22–24 (Tables 58.1 and 58.2). For example, a MESS of 7 to 10 has been generally proposed to predict amputation.23 However, current injury scoring systems should be used as a guide in management, because they have not been shown to correlate well with eventual functional recovery after limb salvage.25 In a recent systematic review of some of these scoring systems (MESS, the Limb Salvage Index [LSI], the Predictive Salvage Index [PSI], and the Gustilo-Anderson scoring system), a good correlation between MESS and amputation rate was found in only 25% of published data.25 Ultimately, sound clinical judgment and a multidisciplinary team approach considering the patient’s limb injury severity, systemic factors, social factors, rehabilitation potential, and functional recovery should dictate limb salvage or primary amputation.26 Every lower extremity trauma is unique and should be treated accordingly. The main indications for limb salvage include any limb injury in a child and adults with bony and soft tissue loss with intact sensation. In some instances, however, determining limb sensibility is difficult in the acute setting. In such a scenario, débridement, vascular reconstruction, and bony fixation are indicated until definitive insensibility is confirmed. Considerations for primary amputation of open tibial fractures include anatomically complete disruption of the posterior tibial nerve in adults, multilevel trauma to the limb with life-threatening injuries, and crush injury with warm ischemia for longer than 6 hours27–29 (Fig. 58.3, Box 58.1).
Oncologic Defects
Preoperative consultation with the reconstructive surgeon, appropriate preoperative imaging, and multidisciplinary planning is critical in dealing with oncologic defects of the lower extremity. Most lower extremity oncologic resections are electively scheduled, which allows time for assembling the appropriate team, whether this involves skeletal or vascular reconstruction, soft tissue coverage, or a combination of these. The objectives of lower extrem ity tumor reconstruction are similar to those of trauma reconstruction with certain caveats. In tumor reconstruction, neoadjuvant chemotherapy or radiotherapy may be required; in such instances delaying the reconstruction to allow recovery of the tissues from acute radiation injury can be considered. Considering the radiation zone of injury to the local tissues and recipient vessels will impact flap selection and reconstruction. Neoadjuvant irradiation has not been shown to increase acute wound or microvascular complications when combined with free flap reconstruction and is associated with fewer late recipient-site complications than adjuvant irradiation.30 Radiated tissues within the wound bed should be replaced with healthy tissues, and sometimes this requires very large flaps (Fig. 58.4). If radiated tissue is left behind, there is a high likelihood that healing will be compromised. Providing durable and predictable reconstruction is critical to allow for wound healing and adjuvant chemotherapy, radiotherapy, or both for locoregional control. Final tumor margins should be confirmed before definitive coverage. A short period of temporary coverage may be needed in certain tumor types until permanent pathologic margins are confirmed, and only then is definitive coverage warranted. Patients with cancer are often malnourished. Nutritional markers such as transferrin, albumin, and prealbumin are useful in determining the need for supplemental nutrition before reconstructive surgery.
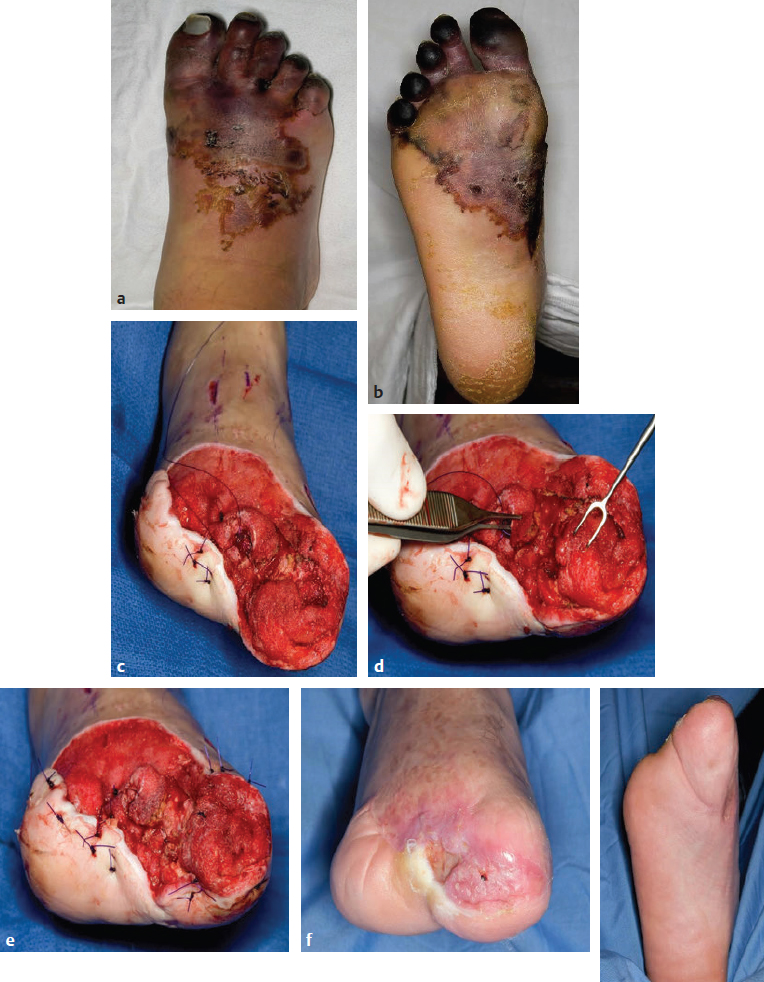
Fig. 58.3 Forefoot reconstruction using local flap options. This 47-year-old man sustained a crush injury to his right foot that resulted in multiple fractures of the metatarsals and phalanges. (a,b) After the area of soft tissue necrosis in the forefoot and midfoot had fully demarcated, he underwent débridement. (c) After partial amputation and wound vacuum therapy. Additional shortening of the proximal phalanx of the great toe was performed. The metatarsophalangeal joint was preserved and the flexor hallucis brevis muscle attachment was maintained to allow for a stable sesamoid–plantar–foot complex. The third and fourth metatarsal necks were further shortened. (d,e) Local tissue with overlying granulation were elevated to the cover stumps of the first digit proximal phalanx and second and third metatarsal bone stumps, and the plantar skin was advanced dorsally. (f,g) Early postoperative result after skin grafting. A free flap was not necessary.
Absolute
• Posterior tibial nerve transection in adults
• Crush injury with warm ischemia >6 hours
• Total leg amputation in adults
• Nonreparable devascularization
Relative
• Life-threatening multisystem trauma
• Severe ipsilateral foot trauma
• Unable to rehabilitate
• Elderly with previous history of advanced diabetes and/or peripheral vascular disease
Source: From Pu Lee LQ, Levine JP, Wei FC. Overview of lower extremity reconstruction. In: Pu LQ, Levine JP, Wei FC, eds. Reconstructive Surgery of the Lower Extremity. St Louis, MO: CRC Press, 2013.