Liver and Kidney Donation Following Cardiac Death
Roberto Hernandez-Alejandro
Lisa Taylor VanHouwelingen
Kris P. Croome
DEFINITION
Donation after cardiac death (DCD) is defined as planned withdrawal of ventilatory and organ-perfusion support (life support) in the face of irreversible brain injury that does not meet the criteria of neurologic death. DCD donors progress to death with a gradual reduction and ultimate irreversible cessation of circulation prior to certifying death. DCD was formerly called non-heart-beating donation (NHBD).
DCD has been categorized according to the Maastricht classification1:
Type 1: dead on arrival
Type 2: unsuccessful resuscitation
Type 3: cardiac arrest after withdrawal of life support in patients who do not meet the criteria of brain death
Type 4: cardiac arrest after brain death
Type 5: unexpected arrest in intensive care
In the vast majority of cases, liver allografts are procured from controlled donors (types 3 and 4). This chapter refers to liver allografts from controlled donors (types 3 and 4).
BACKGROUND
Approximately 15% to 20% of patients die or become too sick to survive transplant while on the waiting list in North America.2
The majority of kidney and liver allografts used in North America are derived from donation after brain-dead donors. In order to augment the limited organ supply, many transplant programs procure livers from DCD donors. Over the last decade, there has been a significant increase in DCD in North America.3
In brain-dead donors, death is certified using neurologic criteria as defined by the Ad Hoc Committee of the Harvard Medical School in 1968. With donors after brain death (DBD) donation, circulation and oxygenation are maintained prior to and during organ recovery. In contrast, controlled DCD involves planned withdrawal of ventilatory and organ-perfusion support (life support) in patients with irreversible brain injury that does not meet the criteria for neurologic death.
During the agonal period following withdrawal of life support, hypoxia and hypoperfusion result in organ injury.
DONOR SELECTION
Donor selection using strict criteria is important for successful transplant outcomes. In liver transplantation, use caution when considering organs from donor older than 40 to 50 years of age or those with higher (>35 kg/m2) body mass index (BMI). Moreover, limit procurement to otherwise “low-risk” allografts. More liberal criteria for selection of kidneys from DCD donors is acceptable, although DCD extended criteria donor kidneys are associated with inferior short- and long-term outcomes.
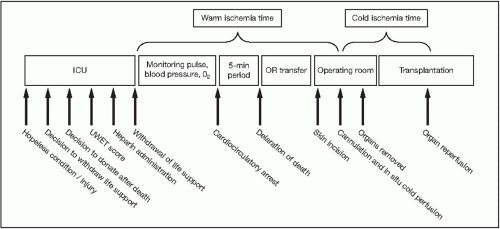
ISCHEMIC TIME
Considering that DCD grafts are high risk, all efforts should be made to minimize ischemic times.
Warm ischemia time in the donor above 35 minutes showed a significant 1.8-fold increase in graft loss rate when compared with warm ischemia time less than 15 minutes.6
The American Society of Transplant Surgeons recommends warm ischemia time for the liver be less than 20 to 30 minutes for the donor.7 Warm ischemia times up to 90 minutes are acceptable in kidney transplantation.
The duration of systolic blood pressure (SBP) less than 50 mmHg is directly correlated with increased rates of diffuse biliary ischemia, delayed renal allograft function, graft loss, and death.8
In liver transplantation, every hour after 6 hours of cold ischemia time led to an increase of 6% in the relative risk for graft failure.
RECIPIENT SELECTION
In a review of the Scientific Registry of Transplant Recipients data for DCD liver transplants, significant recipient factors predicting graft failure were the following6:
Recipient age older than 54 years, male gender, African American race, metabolic disorder, model for end-stage liver disease (MELD) score more than 34 at transplantation, and the need for life support at the time of transplant
Some data suggests hepatitis C virus (HCV) recurrence is more severe in HCV+ recipients who receive grafts from DCD donors compared to those who receive grafts from brain-dead donors.9
Recipients of DCD renal allografts are generally older and have more comorbidities.
PREOPERATIVE PLANNING
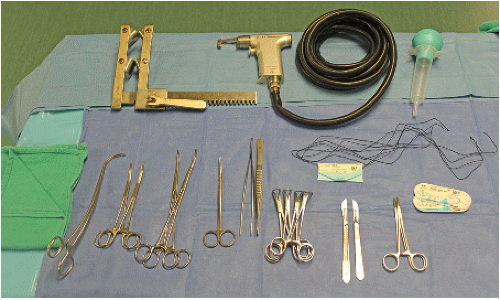
Sequence of events is discussed with scrub nurses and other members of surgical team.
Instruments required for rapid entry and cannulation are chosen (FIG 2).
If withdrawal of support is not performed in operating room (OR)
Operative table height is measured and matched to intensive care bed height.
A pathway from the area of the withdrawal to the OR is cleared.
Heparin (300 units/kg intravenously [IV]) should be administered, prior to withdrawal of support, if allowed. This decision is based on institutional protocol. If pre-withdraw heparin is not allowed, heparin should be added to the preservation fluid.
Operating team scrubs once arrest has been declared.
A minimum waiting time between arrest and declaration of death must be observed and will depend on local practices (2 to 10 minutes).
The procurement surgeon should not be involved in any aspect of clinical decision making during the time of withdrawal of care and declaration of death.
TECHNIQUES
The objective is to cannulate major vessels as quickly as possible to perfuse the liver in situ with cold preservation solution.
Proviodine prep solution should be used from the pubis to the chin.
A midline skin incision is made (this can be extended to a cruciate skin incision for exposure).
Hold the skin flaps open with piercing towel clips, or a large retractor.
Retract the right colon and small bowel to the left using a surgical towel.
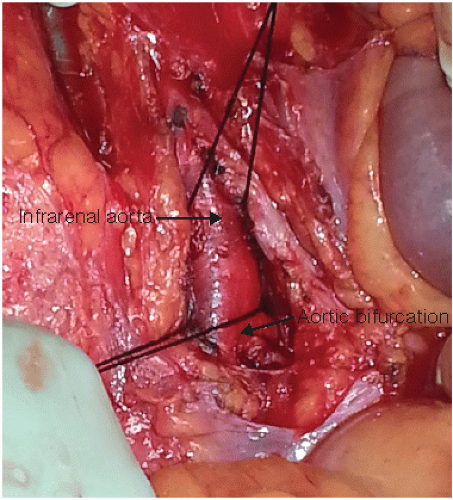
Complete a right medial visceral rotation (Cattell-Braasch maneuver).
Encircle the aorta with a right-angle clamp. A no. 4 silk is looped proximally and the aorta is clamped with a Kelly clamp distally or encircled (FIG 3).
Cannulate the aorta through an aortotomy and flush with cold preservation solution. Start the flush immediately (FIG 4A,B).
There is some debate as to the optimal perfusion solution to use with DCD organ procurement. The authors prefer a low-viscosity fluid in an effort to optimally perfuse the terminal arterioles and remove microthrombi.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree