Introduction
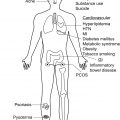
Hidradenitis suppurativa (HS) is a chronic inflammatory disorder with a profoundly negative impact on quality of life in patients with active disease. Many lifestyle factors are associated with disease incidence, severity, and exacerbation. This chapter addresses potentially modifiable lifestyle factors that may affect disease activity, including diet, obesity, tobacco use, and psychological stress.
Diet
Emerging evidence indicates that dietary modifications may be a viable adjunctive therapy for HS. More than 75% to 90% of HS patients have made dietary changes in an attempt to manage HS symptoms, altering at least one food item from their diet (gluten, dairy, and refined carbohydrates were the most common). Half to two-thirds of HS patients perceive improvement from dietary changes, while less than 5% reported worsening of HS.
Though dietary changes are very common in HS, evidence supporting dietary recommendations in HS is weak to moderate at best. There is a dearth of literature evaluating the impact of dietary choices on HS severity, and no randomized controlled studies. The little that is known about diet in HS comes from several small studies, and support for dietary recommendations in HS is frequently extrapolated from the literature on acne, due to its pathologic similarities to HS.
Mechanism of Diet in Follicular Occlusion
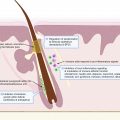
The proposed initiating event in the pathogenesis of HS involves occlusion of the folliculopilosebaceous unit. Keratotic debris accumulates in the occluded follicle, eventually leading to follicular rupture and triggering an inflammatory cascade. Diet, particularly dairy products and simple carbohydrates, may contribute to follicular occlusion through hormonal or other cellular signaling pathways ( Fig. 27.1 ).
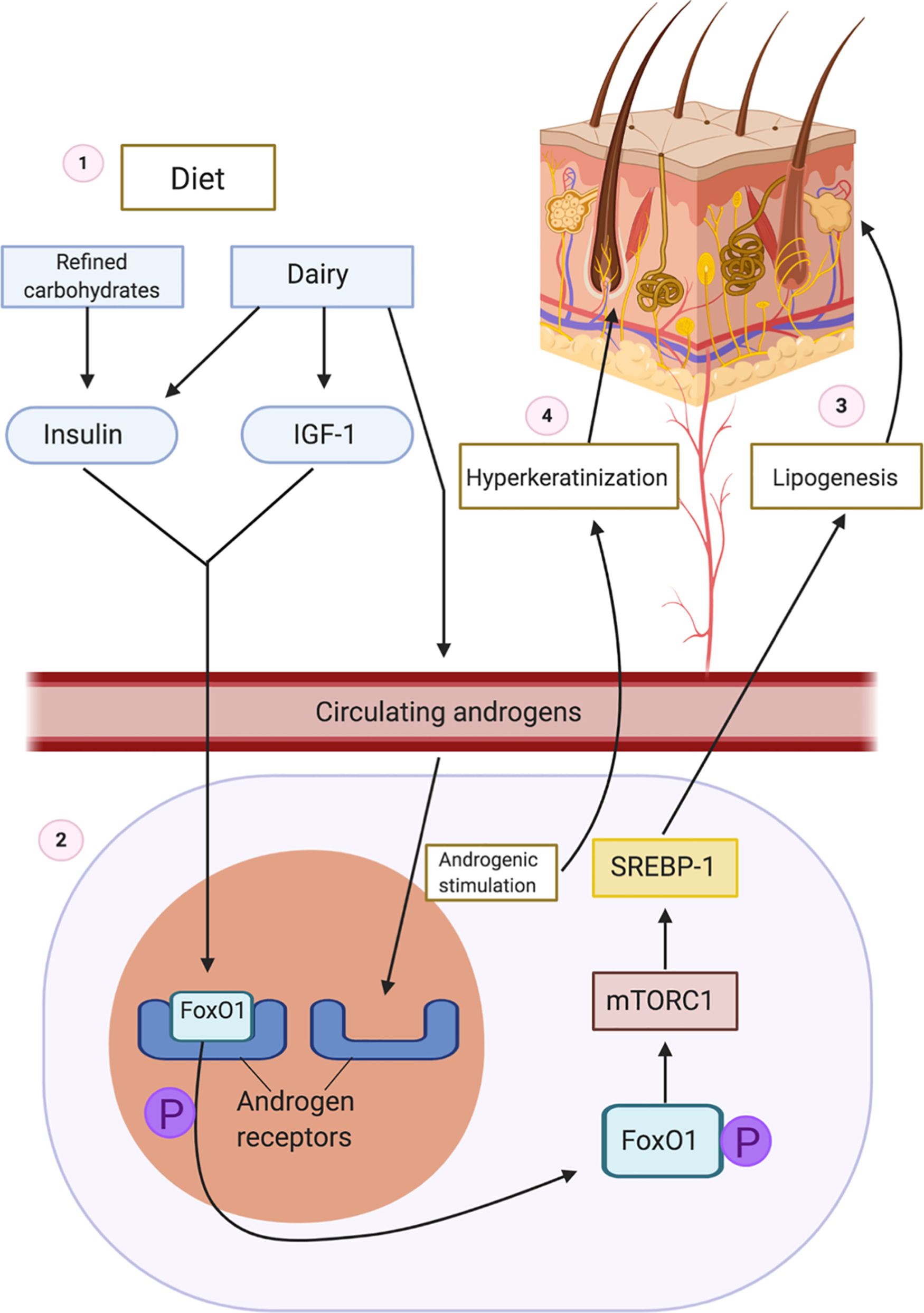
- 1.
Ingestion of carbohydrates increases insulin. Dairy increases insulin, IGF-1, and circulating androgens.
- 2.
FoxO1 is normally bound to the androgen receptor to suppress androgenic signaling. Increased insulin and IGF-1 levels lead to phosphorylation of nuclear FoxO1, which causes subsequent expulsion of FoxO1 from the nucleus to the cytoplasm.
- 3.
In the cytoplasm, FoxO1 stimulates mTORC, which acts on STEBP to increase lipogenesis.
- 4.
Circulating endogenous and exogenous androgens bind to androgen receptors, leading to hyperkeratinization of the hair follicle which eventually ruptures.
Dairy products increase insulin and insulin-like growth factor 1 (IGF-1) levels, and refined carbohydrates increase insulin levels, leading to hyperinsulinemia. Normally, forkhead box transcription factor O1 (FoxO1), the gatekeeper in the pathogenesis of acne, suppresses androgen receptor activation in the nucleus. Hyperinsulinemia and elevated levels of IGF-1 lead to phosphorylation and subsequent expulsion of FoxO1 from the nucleus to the cytoplasm, where it no longer suppresses androgen receptors. Without nuclear FoxO1, androgen receptors are exposed and susceptible to binding circulating androgens. In addition to suppressing androgenic signaling, FoxO1 regulates immune responses, modulates regulatory proteins that control sebaceous lipogenesis, opposes oxidative damage, and regulates mTORC1 (the kinase mammalian target of rapamycin complex 1). mTORC1 regulates cellular metabolic activity, growth, and proliferation. Cytoplasmic FoxO1 indirectly activates the mTORC1 signaling pathway, which acts on sterol regulatory binding protein (SREBP-1), inducing lipogenesis in sebaceous (oil-producing glands) in acne. Because dairy and refined carbohydrates can contribute to the dysregulation of FoxO1 and mTORC1, limiting consumption of these items may be beneficial in treating acne. Elevated levels of IGF-1 and increased cytoplasmic expression of FoxO1 have been observed in acne, and given the pathologic similarities between acne and HS, these dietary interventions may also be beneficial in HS.
Additionally, cow’s milk often contains androgenic hormones. The increased propensity to have open androgen receptors along with the addition of exogenous androgens from dairy products can lead to excessive androgenic activation and subsequent follicular hyperproliferation seen in acne. Due to androgenic stimulation, the hair follicle becomes occluded with poorly differentiated keratinocytes in HS, contents accumulate in the hair follicle, and it eventually ruptures. Androgens such as progesterone, estrogen, and testosterone have been hypothesized to affect HS disease activity, although the effects of each hormone have yet to be elucidated. While the exact role of androgens in HS is poorly understood, it appears that ingestion of dairy and refined carbohydrates may contribute to an overactive androgenic response, which exacerbates follicular occlusion and perpetuates the inflammatory cascade triggered by follicular rupture.
Mechanism of Diet in Inflammation
Inflammation is key to the development of active disease in HS. The majority of our current medical interventions for HS target inflammation to attenuate disease activity. Patients with HS often have co-morbidities, such as metabolic syndrome and obesity, which produce states of chronic non-specific inflammation. Diet is a potentially important source of pro- and anti-inflammatory nutrients ( Table 27.1 ). The specific type of macronutrients and their relative amounts play a role in regulating inflammation. This section reviews scientific data on impact of carbohydrates, fats, and sodium on inflammation. While there are case reports of worsening acne after consumption of supplemental whey protein, its effect on HS is unknown.
| Nutrient | Response |
|---|---|
| Carbohydrates | |
| Hyperglycemia: PRO-inflammatory | Increases non-specific inflammation by triggering pro-inflammatory NF-κB activation in monocytes |
| Postprandial elevated glucose levels trigger macrophage production of IL-1β and insulin. Insulin then enhances macrophage production of IL-1β, in a positive inflammatory feedback loop of insulin and IL-1β, resulting in chronic inflammatory cycle | |
| Hyperinsulinemia: PRO-inflammatory | Increased non-specific inflammation, promotes omega-6 metabolism, increasing inflammatory eicosanoids |
| Increases levels of advanced glycation end-products (AGEs) with hyperglycemia: PRO-inflammatory | Increased non-specific inflammation via multiple signaling pathways, increase TNF-α and IL-1 Promote increased levels of androgens and insulin in women with PCOS |
| Fiber: ANTI-inflammatory | Reduces glycemic index of carbohydrates thus decreases level of hyperglycemia |
| Improves gut microbiome diversity with decreases in inflammation Healthy gut microbiome modulates systemic Treg/Th17 balance with decrease in non-specific inflammation | |
| Whole vegetables: ANTI-inflammatory | Antioxidants, block reactive oxygen species and thus decrease inflammation triggered by ROS |
| Dampen inflammatory response by increasing threshold for activation of NF-κB and reducing pro-inflammatory cytokines | |
| Lipids | |
| Saturated fats: PRO-inflammatory | Activates inflammasome, increases production of IL-1β Decreases microbiome diversity |
| Omega 6 fatty acids: PRO-inflammatory | Metabolism leads to Arachidonic acid and pro-inflammatory eicosanoids |
| Omega 3 fatty acids: ANTI-inflammatory | Metabolism leads to DHA/EPA and production of anti-inflammatory eicosanoids Production of resolvins and protectins with resolution of inflammatory cascade |
| Proteins | |
| Mammalian meats: PRO-inflammatory | Contain Arachidonic acid If cooked at high temperatures (grilling) lead to production of AGEs |
| Dairy/eggs: PRO-inflammatory | Contain Arachidonic acid |
| Fish, fatty cold temperature: ANTI-inflammatory | Contain anti-inflammatory omega-3 Fatty acids |
| Electrolytes | |
| Sodium chloride: PRO-inflammatory | Reduce activation of IL-4 and IL-13 stimulated macrophages |
| Alters gut microbiome Induces Th17 differentiation | |
Carbohydrates
Consumption of refined and excessive carbohydrates can lead to chronically elevated glucose levels. Hyperglycemia increases circulating cytokines such as interleukin (IL) 6, IL-18, and tumor necrosis factor alpha (TNF-alpha). Chronic hyperglycemia leads to chronic hyperinsulinemia, which prompts increased metabolism of omega-6 fatty acid in production of arachidonic acid and its downstream inflammatory components. In addition, hyperglycemia can increase nuclear factor κB (NF-κB) activation in monocytes, increasing levels of pro-inflammatory TNF-alpha. Elevated glucose levels after eating induce macrophages to secrete IL-1 beta, which in turn promotes postprandial inflammation.
Carbohydrate ingestion results in production of advanced glycation end products (AGEs), which are formed by the non-enzymatic reaction between reducing sugars and proteins, lipids, and DNA. AGEs can be increased by either endogenous or exogenous sources. Endogenous sources of AGEs are secondary to chronic hyperglycemia, while exogenous sources are found in foods processed at high temperatures, including commercially processed carbohydrates. AGEs trigger increases in NF-κB and as such are pro-inflammatory. In addition, AGEs have hormonal effects leading to elevations in testosterone, insulin, and reactive oxygen species in women with polycystic ovary syndrome. These pro-inflammatory effects of hyperglycemia can be detrimental to health and immunity.
Fat
While omega-3 and omega-6 fatty acids are both essential nutrients, omega-3 fats are anti-inflammatory and omega-6 fats are pro-inflammatory. Through the arachidonic acid pathway, omega-6 fats act as precursors for the production of pro-inflammatory eicosanoids. While the ideal ratio of omega-6 fats to omega-3 fats is unknown, it is hypothesized that a ratio greater than 10:1 promotes inflammation. It is thought that humans evolved consuming a 1:1 ratio of omega-6:omega-3 fats. Our current typical pro-inflammatory Western diet (high in refined sugars, meats, processed foods, and fat) has an elevated pro-inflammatory omega-6 to omega-3 ratio of approximately 15:1. Saturated fats, often present in animal products and baked goods, also exert pro-inflammatory effects.
Sodium
Processed foods are frequently high in saturated fats, refined carbohydrates, and sodium. Not only does excessive sodium intake contribute to hypertension, but high salt intake may inhibit proper immune cell function. Excessive salt intake alters the intestinal microbiome (depleting beneficial bacterial species important for gut health and immunity) and promotes a pro-inflammatory T helper (Th) 17 phenotype. High dietary salt intake also suppresses anti-inflammatory macrophage activity in mouse models, reducing noninflammatory innate immune cell activation, which may lead to an overall imbalance in immune homeostasis.
Dietary Considerations in Hidradenitis Suppurativa
Multiple reports on restrictions of specific dietary components are present in HS literature. Most notable are reports on limiting dairy and brewer’s yeast. Nightshades have been addressed as a potential dietary trigger for HS, and intermittent fasting as an intervention has also been reported. Potential benefits of the Mediterranean diet in HS have also been described.
Dairy
Dairy products include milk as well as products made from milk, such as cheese, yogurt, and cottage cheese. Eggs are typically not considered to be dairy products as they are not milk-based. Dairy is hypothesized to contribute to follicular occlusion in HS by way of exogenous hormones and hyperinsulinemia. Danby et al. reported that of 47 patients who followed a dairy-free diet, 83% improved while none reported worsening symptoms. Another study revealed that patients who eliminated dairy were twice as likely to report HS improvement as those who did not. Of the limited available evidence, it appears the reduction or elimination of dairy may be one of the more effective dietary recommendations in HS. However, because dairy is an important dietary source of calcium, patients following a dairy-free diet may require supplemental calcium and vitamin D.
Brewer’s Yeast
Brewer’s yeast (also called baker’s yeast) has been implicated as a dietary trigger in HS. Brewer’s yeast contains Saccharomyces cerevisiae , a fungus that ferments alcohol and allows bread products to rise. A small study was conducted in 12 HS patients who followed a wheat and brewer’s yeast-free diet for 12 months postoperatively after surgical excision of HS lesions. Patients were instructed to eliminate all baked products, vinegar, soy sauce, beer, wine, fermented cheeses, and mushrooms. All patients experienced regression of HS lesions post-operatively while on the brewer’s yeast-free diet, and all reported recurrence of skin lesions with reintroduction of wheat or yeast, and subsequent regression after removing these products from the diet again. Study participants also reported improved quality of life after surgical and dietary intervention. While elimination of brewer’s yeast shows disease-attenuating potential, this study was done in conjunction with surgical excision, making it difficult to distinguish between the benefit of surgery and elimination of brewer’s yeast.
Glycemic Load
Low-glycemic-load diets have not been studied in HS, but they have been investigated in acne. Glycemic load refers to the amount of carbohydrate consumed multiplied by the rate at which the carbohydrate is metabolized and enters the bloodstream (glycemic index). A study conducted in male acne patients comparing a low-glycemic-load diet to a carbohydrate-dense diet showed a greater decrease in total number of acne lesions in the low-glycemic-load group compared to the control group. In addition to improvement in acne, the low-glycemic-load diet resulted in greater weight reduction and improvement in insulin sensitivity than the control diet. While acne and HS have a similar pathogenesis, HS primarily affects women, and this study was conducted using only male subjects, so results are not necessarily generalizable to HS patients. Still, there may be some theoretical benefit to a low-glycemic-load diet in HS, as high carbohydrate intake can lead to hyper-insulinemia, which may exacerbate follicular occlusion and inflammation.
Nightshades
Nightshades, plants from the Solanaceae family, typically contain high amounts of alkaloids, which have the potential to be toxic. Tomatoes, potatoes, eggplants, peppers (both bell and hot), and tobacco are included in this group. While discussions on HS internet platforms reveal that some HS patients feel nightshades exacerbate HS, evidence is lacking. Current literature indicates that HS symptom improvement is not significant after elimination of peppers or eggplants. Those who eat tomatoes are more likely to experience HS improvement than those who do not. While the elimination of nightshades may be a common practice among HS patients, it is not well-supported by evidence.
Fasting
The impact of intermittent fasting has been evaluated in a study of 55 patients with HS who fasted (abstained from food and beverages from sunrise to sunset) for one month during Ramadan. Mean International Hidradenitis Suppurativa Severity Score System (IHS4) scores from before and after Ramadan decreased significantly. The greatest changes in IHS4 score were observed in those receiving topical antibiotics followed by systemic antibiotics, and the smallest changes were in those receiving biologics. Mean weight change during Ramadan was 0 kg, indicating that score changes were not related to weight loss. By the end of Ramadan, abscesses and draining fistulas decreased for 69% and 37.5%, respectively. However, of those that improved, the improvement only persisted in one-fourth of those patients at one month post completion of fasting. As the disease recurred with reinstitution of normal dietary patterns, all changes were likely dependent on persistent intermittent fasting, which may be impractical for most patients. The authors hypothesized that study results could be due to a fasting-induced downregulation of Th1, Th17, and antigen-presenting cells, as intermittent fasting has been shown to affect the composition of T cells in the gut with a reduction of IL-17-producing T cells and increased numbers of regulatory T cells. Additionally, intermittent fasting has been shown to increase gut microbiome diversity and activate microbial metabolic pathways that modulate systemic immune responses.
Mediterranean Diet
In a cross-sectional study, Barrea et al. evaluated how closely dietary intake from 7-day food records resembled the Mediterranean diet in 41 HS patients and 41 healthy controls. This study revealed that, despite comparable total caloric consumption, HS patients consumed lower amounts of complex carbohydrates, monounsaturated fat, and omega-3 polyunsaturated fats, and higher amounts of saturated and omega-6 fats than healthy controls. Those consuming diets with poor resemblance to the Mediterranean diet tended to have more severe HS than those consuming a more Mediterranean-style diet. HS severity was also positively correlated with both total and simple carbohydrate intake, and negatively correlated with omega-3 fat intake. Authors surmised that these observations could be due to anti-inflammatory properties of the Mediterranean diet, such as antioxidants and polyphenols found in plant foods. Given that consumption patterns reflective of the Mediterranean diet were associated with decreased HS severity, the Mediterranean diet may be a valuable dietary intervention in HS. However, cost may preclude some patients from adhering to this dietary pattern.
Anti-Inflammatory Diet
Certain dietary patterns have been described as anti-inflammatory due to an increase in components that can decrease inflammation while minimizing foods that increase inflammation. The most well-studied diet that is considered anti-inflammatory is the Mediterranean diet; studies have shown that it can reduce mortality, cardiovascular disease, cancer, neurodegenerative diseases, and diabetes. This plant-based diet is high in fruits, vegetables, fiber, beans, lentils, nuts, and omega-3 fats, and low in animal protein and saturated fats. Fruits and vegetables are high in vitamins, minerals, fiber, and phytonutrients that counteract inflammation. Fish is an important source of protein in an anti-inflammatory diet as it is high in omega-3 fats, while meat and dairy products can have high levels of omega-6 fats that promote inflammation via generation of arachidonic acid. Refined, commercially processed carbohydrates are often stripped of nutrients and result in rapid increases in serum glucose and hyperinsulinemia, which contribute to systemic inflammation. Whole-grain carbohydrates retain nutrients and fiber, moderating blood sugar fluctuation and promoting a healthy diverse gut microbiome. When recommending a healthy and potentially anti-inflammatory diet, it is important to recognize that dietary components work synergistically to combat the effects of inflammation. Culinary recommendations for a healthful diet are summarized in Box 27.1 .