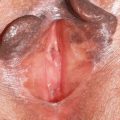
Lichen sclerosus (LS) is an inflammatory skin disease predominantly affecting the anogenital region. If untreated, progressive sclerosis results in scarring with distortion of the normal architecture. LS occurs more commonly in women than men but may occur in all age groups, including adolescents and prepubertal children. Its exact prevalence is unknown, but estimates range from 1:60 to 1:1000. In this article, LS is discussed in detail with respect to disease management in adults and children, risk of malignancy, and association with other diseases.
Disease pathogenesis
Lichen sclerosus (LS) is a complex chronic inflammatory skin disease, with genetic, physiologic, and environmental factors that influence the clinical phenotype and disease outcome. It is generally accepted as an autoimmune disorder with a predilection for genital skin, but the exact target antigen and disease pathogenesis remain controversial. Hallopeau and Darier first described lichen sclerosus at the end of the nineteenth century as a variant of lichen planus (LP). Now LP and LS are generally accepted as different entities on the basis of distinguishing clinical and histologic features, although the diseases overlap. Early stages of LS and LP can sometimes be difficult to differentiate clinically, and established cases of vulvar LS may develop introital LP. This crossover subgroup can be more refractory to treatment.
The exact pathogenesis of LS remains unclear, although clinical observations are leading to a translational medical approach to unraveling disease pathogenesis. LS is considered to be an autoimmune disorder and, in common with other autoimmune diseases, is found more commonly in females. The condition has been associated clinically and immunologically with autoimmune thyroiditis, pernicious anemia, vitiligo, and alopecia areata. These disorders should be considered in the evaluation of patients suspected of having LS and are useful both clinically in the diagnosis of LS and in the detection of other early subclinical autoimmune conditions. From clinical experience, patients with undiagnosed autoimmune thyroiditis may present with LS, which is more difficult to control until they return to a clinically euthyroid state.
The genetic contribution to the development of lichen sclerosus is also complex. Familial cases have been described in identical and nonidentical twins and siblings, although the inheritance pattern is not clearly established. Genes that regulate the human leukocyte antigens (HLA) have been investigated to see if associations between HLA antigens and LS exist. No associations have been found between HLA class 1 antigens, which occur on the surface of all nucleated cells and platelets. LS has been shown to be associated with type 2 antigens particularly DQ7, -8 or -9. HLA type 2 antigens are expressed on immunocompetent cells that normally recognize foreign particles.
Established areas of lichen sclerosus show a T-lymphocyte dominant inflammatory infiltrate, sometimes with an associated lymphocytic vasculitis. When T-cell receptor gene rearrangement studies from affected penile and vulvar skin are performed, a monoclonal population of rearranged γ-chain gene of the T-cell receptor have been identified, which could explain the increased risk of malignant transformation in affected individuals.
Because the inflammation in LS initially targets the basement membrane, resulting in apoptosis of basal cells, it has been postulated that the target antigen may lie in this region. Evidence for this comes from work showing circulating autoantibodies to the endothelial cell adhesion molecule, ECAM-1, in 67% of patients with LS.
Antibodies targeting the basement membrane zone, (chiefly BP 180 and BP 230), have been identified in 30% of sera of affected patients. The mechanism leading to the synthesis of these autoantibodies is not fully understood. Current theories postulate that oxidative stress as a result of DNA damage induced by reactive oxygen species, as a result of chronic inflammation, may lead to the disease and possibly any associated malignancy.
Debate continues with respect to the pathogenic role played by Borrelia burgdorferi infection in triggering LS. In Europe there is some supporting evidence for Borrelia infection and LS from polymerase chain reaction amplification of Borrelia-type genetic material in the patches of LS. This finding has not been replicated in studies from the United States.
It is possible that Borrelia infection may act as one of several environmental triggers that can trigger LS in genetically susceptible individuals. Extragenital LS, occurring as patches that clinically resemble scleroderma, is reported to occur in approximately 6% of patients with vulvar lichen sclerosus. It is known that lesional LS skin has altered extracellular matrix proteins similar to scleroderma, a condition that, in localized cutaneous disease, may coexist and show clinical similarities to LS. Acrodermatitis chronica atrophicans, a sclerodermatous skin reaction to Borrelia, has clinical and histologic features of localized scleroderma, but the lesions are usually acral in distribution rather than genital.
Finally, because the 2 peaks in incidence for vulvar LS occur during times of low estrogen, before puberty and after menopause, there is some debate as to the exact role played by hormones in disease pathogenesis. In normal female genitals, the transition from vagina to vulva is marked by an increase in androgen receptors and a decrease in estrogen and progesterone receptors. Vulvar androgen receptor expression seems to be decreased in a subgroup of individuals with LS. Work has been recently published that suggests that disturbance of androgen-dependent growth of the vulvar skin by oral contraceptive pills (OCPs), and especially OCP with antiandrogenic properties, might trigger the early onset of LS in a subgroup of susceptible young women.
Therefore, lichen sclerosus is a complex disease expressed in individuals who are at increased risk of autoimmune disease. External triggers that may influence the exact timing of disease onset might include oxidative stress from chronic inflammation, hormonal manipulation from the use of oral contraceptive agents, and possibly even infection. The clinical features of the disease, treatment options, failures, and disease complications are discussed next.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree