Left Lobe Living Donor Liver Transplantation in Adults
Jean F. Botha
DEFINITION
Left lobe living donor liver transplantation (LDLT) is the implantation of a left hepatic lobe procured from a living donor into a suitable recipient. Left lobe LDLT is associated with the additional risk of the development of small-for-size syndrome (SFSS) and may require operative measures to modulate portal blood flow into the allograft.
In one of the early series of left lobe recipients in whom the graft-to-recipient weight ratio (GRWR) was less than 0.8, graft survival at 3 months was 54%.
Experience over the last two decades has led to a better understanding of the factors implicated in the pathogenesis of SFSS and the successful application of techniques to avoid portal hyperperfusion and SFSS.
With donor safety being the overriding concern and the relatively lower risks associated with left lobe donation combined with the technical refinements and use of graft inflow modulation, it could be argued that, whenever possible, the left lobe should be the graft of choice for LDLT.
PATIENT HISTORY AND PHYSICAL FINDINGS
Potential living donor liver transplant recipients should all undergo a thorough transplant evaluation and are required to be listed on the cadaveric waiting list prior to being accepted for living donor transplant. Living donor liver transplants can only be performed at accredited transplant centers. Accreditation mechanism varies by country. As donors have no medical need for the operation, donor safety is of utmost concern.
IMAGING AND OTHER DIAGNOSTIC STUDIES
Because of the possible need to create a hemiportacaval shunt in the recipient, all patients undergo either a magnetic resonance imaging (MRI) or triple-phase computed tomography (CT) scan of the abdomen in order to document patency of the main, right, and left portal vein (LPV).
SURGICAL MANAGEMENT
Preoperative Planning
Accurate size-matching between donor and recipient is essential. Volumetric assessment of the potential left lobe is needed in order to estimate the GRWR. From the recipient standpoint, a GRWR of 0.8 is desirable; however, we believe a GRWR down to 0.6 is acceptable.
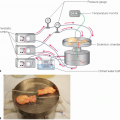
Radiologic software products that provide a three-dimensional model of the liver assist in preoperative planning and assessment of graft weight (FIG 1).
Positioning
Place the patient in the supine position, with the right arm extended at 90 degrees and the left arm placed at the side.
Place two arterial lines as well a 9-Fr central venous catheter for invasive monitoring and rapid fluid infusion.
Place a nasogastric tube and a Foley catheter.
Avoid hypothermia by close monitoring of the ambient temperature as well as the active warming of the patient with hot air blankets.
TECHNIQUES
Make a bilateral subcostal incision to enter the abdominal cavity and divide the round ligament and the falciform ligament. Use a sturdy, table-mounted retractor system.
HILAR DISSECTION
Dissect the proper hepatic artery. Expose and individually ligate the left and right branches. This is to provide as many options as possible for arterial reconstruction and appropriate size-matching to the donor left hepatic artery.
Divide the common bile duct as high as possible in the hilum so that a duct-to-duct biliary anastomosis may be possible.
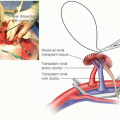
Dissect the portal vein exposing circumferentially both left and right portal branches, so that a right portal to inferior vena cava (IVC) (hemiportacaval) shunt can be constructed, if necessary.
MOBILIZATION OF THE LIVER AND CAVAL PRESERVATION
The recipient IVC needs to be preserved. If technically feasible, this can be done before clamps are placed. We find that it is easier to fully mobilize the retrohepatic IVC and place clamps on the IVC above and below the liver.
Place a vascular clamp across the main portal vein and divide the right and left branches of the portal vein.
With the liver fully clamped, the liver is dissected from the IVC up to the hepatic veins. Suture ligate small branches to the IVC. Take care when dividing the hepatic veins, particularly the common orifice of the left and middle hepatic vein.
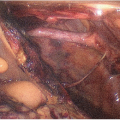
Place a Satinsky clamp across the hepatic vein orifices, or the caval clamps can remain in place for the hepatic venous anastomosis (FIG 2).
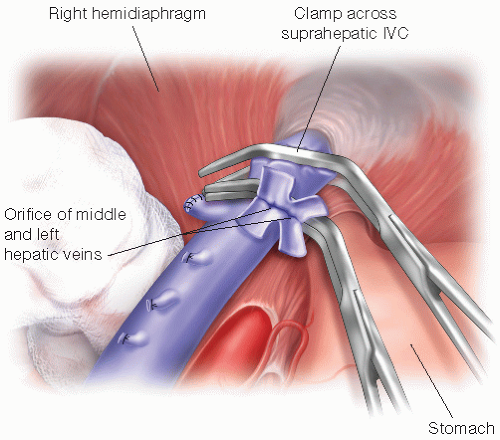 FIG 2 • The upper abdomen after removal of the diseased liver with clamp placed across the IVC and the orifice of the middle and left hepatic veins having been prepared for the hepatic venous anastomosis.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|