Cutaneous injuries that result in scar formation are relatively common, leading patients to seek treatment for cosmetic or functional improvement. Treatments that have the potential to improve or eliminate scarring include radiation therapy, surgical excision, and intralesional injections of corticosteroids, 5-flourouracil, or bleomycin. Unfortunately, these methods are associated with high recurrence rates and untoward sequelae such as skin atrophy, dyspigmentation, and pain. Laser scar revision is a safe procedure with clinically demonstrable efficacy and minimal side effects when used alone or in combination with other scar treatments. The specifics of current laser scar revision techniques are addressed in this overview.
- •
An imbalance in wound-healing homeostasis is at the epicenter of scar formation
- •
Scars, particularly facial scars, significantly affect the lives of patients
- •
Physicians can improve the quality of patients’ lives via scar revision
- •
Numerous scar treatments are available, but lasers have proven to deliver the most reproducibly good results
The psychosocial impact of cutaneous scarring can be profound. Scars inflicted by traumatic incidents, surgical procedures, and severe acne bear a heavy emotional burden on patients, particularly when present on visible areas such as the face. The quality of life of patients may be affected from aesthetic concerns in addition to chronic symptoms such as pruritus and pain. In addition, substantial anxiety and self-consciousness has been noted in men and women when trauma or elective procedures result in even nominal scarring. Cutaneous injuries that result in scar tissue formation are relatively common and lead patients to seek treatment for cosmetic or functional improvement. It is imperative for physicians to recognize that physical improvement of scars can translate into improved psychosocial well-being and behavior of patients.
Scars are the result of a deviation in the orderly pattern of healing and can be caused by a variety of factors, such as excessive wound tension, improper surgical repair, delayed reepithelialization, or a history of radiation to the affected area. The underlying pathophysiologic mechanism appears to be an imbalance of matrix degradation and collagen biosynthesis. An overzealous healing response can create a raised nodule of fibrotic tissue, whereas “pitted” and atrophic scars may result from inadequate replacement of deleted collagen fibers. Although vascular and pigment alterations associated with wound healing are typically transient, the textural changes caused by collagen disruption are often permanent. Histologically, what makes scars unique is the relative absence of skin appendages and elastic fibers—constituents of normal skin that may account for the loss of flexibility seen in scar tissue.
There are several currently available scar-reducing therapies and many other agents that may emerge to have the potential to eliminate scarring. Some of the most commonly used modalities to improve scar appearance include intralesional corticosteroids, 5-fluorouracil, and bleomycin. It is likely that these agents exert their effect on scars by suppressing inflammation and/or collagen production. Combination therapy is often advocated as a means of increasing efficacy and decreasing total medication dosage, thereby decreasing the likelihood of adverse effects. Radiation therapy and surgical intervention, particularly in combination with one another, are sometimes used for refractory and recurrent scars. Unfortunately, each of these methods has been associated with unacceptably high incidences of scar recurrence and other untoward sequelae such as skin atrophy, dyspigmentation, and pain. Laser scar revision is a safe procedure with clinically demonstrable efficacy and minimal side effects that may be used in combination with the aforementioned scar treatments. The remainder of this article addresses the use of lasers for the treatment of scars.
History of laser scar revision
- •
The principles of selective photothermolysis help guide the laser surgeon in choosing the proper laser wavelength and treatment parameters for scar revision
- •
Proper scar classification is essential for optimizing treatment results
Although laser surgery is more than 5 decades old, the field was revolutionized in 1983 when Anderson and Parrish elucidated the principles of selective photothermolysis. This basic theory of laser-tissue interaction explains how selective tissue destruction is possible. To effect precise thermal destruction of target tissue without unwanted conduction of heat to surrounding structures, the proper laser wavelength must be selected for preferential absorption by the intended tissue chromophore. Furthermore, the pulse duration of laser emission must be shorter than the thermal relaxation time of the target, thermal relaxation time (T R ) being defined as the amount of time necessary for the targeted structure to cool to one-half of its peak temperature immediately after laser irradiation. The delivered fluence (energy density) must also be sufficiently high to cause the desired degree of thermal injury to the skin. Thus the laser wavelength, pulse duration, and fluence must each be carefully chosen to achieve maximal target ablation while minimizing surrounding tissue damage.
Laser systems are versatile tools that allow for a broad range of cutaneous maladies to be treated. Scar improvement with a pulsed dye laser (PDL) was first reported in 1993, and over the past decade laser scar revision has progressed tremendously, due to advances in technology.
Laser treatment of scars is optimized by proper scar categorization. Several qualities of the scar including size, color, texture, and prior treatments influence choice of laser wavelength and treatment parameters.
Scar classification
- •
Categorization of scars by clinical appearance can be difficult, but is a helpful guide to proper laser treatment
- •
Although similar in many respects, hypertrophic scars and keloids should be distinguished from one another to optimize clinical outcome
- •
Atrophic scars are dermal depressions and result in significant contour abnormalities
- •
Recognition of skin prone to scarring is one tool of evaluation that can be helpful in scar prevention
Numerous scar classification system and evaluation tools have been described. At present, no universal model for objective scar assessment has been accepted. In medical literature, scars are often analyzed by their etiology, the most common sources being surgery, trauma, burns, and acne or inflammatory processes. For the purposes of practicality and ease in treatment selection, the authors advocate scar classification as determined by clinical appearance rather than by causation.
Hypertrophic scars are erythematous, raised, firm nodular growths that occur more commonly in areas subject to increased pressure or movement or in body sites that exhibit slow wound healing. The growth of hypertrophic scars is limited to the site of original tissue injury, unlike keloids , which proliferate beyond the boundaries of the initial wound and often continue to grow without regression. Keloids present as deep reddish-purple papules and nodules, often on the earlobes, anterior chest, shoulders, and upper back. These lesions are more common in darker-skinned persons and, like hypertrophic scars, may be pruritic, dysesthetic, and cosmetically disfiguring. Whereas the histology of hypertrophic scars is indistinguishable from that of other scarring processes, keloidal histology may be recognized by thickened bundles of hyalinized acellular collagen haphazardly arranged in whorls and nodules with an increased amount of hyaluronidase.
Atrophic scars , on the other hand, are dermal depressions that result from an acute inflammatory process affecting the skin, such as cystic acne or varicella. The inflammation associated with atrophic scars leads to collagen destruction with dermal atrophy. Surgery or other forms of skin trauma may also result in atrophic scars, which are initially erythematous and become increasingly hypopigmented and fibrotic over time. Based on their width, depth, and 3-dimensional architecture, acne scars are sometimes further subclassified into icepick, rolling, and boxcar scars.
Prescars are early wounds in scar-prone skin. Prophylactic or early laser treatment of traumatized skin concomitant with or shortly after cutaneous wounding has been shown to reduce or even prevent scar formation in patients at high risk for scarring. Laser therapy may improve the appearance of wounded skin by promoting better collagen organization in healing wounds.
Scar classification
- •
Categorization of scars by clinical appearance can be difficult, but is a helpful guide to proper laser treatment
- •
Although similar in many respects, hypertrophic scars and keloids should be distinguished from one another to optimize clinical outcome
- •
Atrophic scars are dermal depressions and result in significant contour abnormalities
- •
Recognition of skin prone to scarring is one tool of evaluation that can be helpful in scar prevention
Numerous scar classification system and evaluation tools have been described. At present, no universal model for objective scar assessment has been accepted. In medical literature, scars are often analyzed by their etiology, the most common sources being surgery, trauma, burns, and acne or inflammatory processes. For the purposes of practicality and ease in treatment selection, the authors advocate scar classification as determined by clinical appearance rather than by causation.
Hypertrophic scars are erythematous, raised, firm nodular growths that occur more commonly in areas subject to increased pressure or movement or in body sites that exhibit slow wound healing. The growth of hypertrophic scars is limited to the site of original tissue injury, unlike keloids , which proliferate beyond the boundaries of the initial wound and often continue to grow without regression. Keloids present as deep reddish-purple papules and nodules, often on the earlobes, anterior chest, shoulders, and upper back. These lesions are more common in darker-skinned persons and, like hypertrophic scars, may be pruritic, dysesthetic, and cosmetically disfiguring. Whereas the histology of hypertrophic scars is indistinguishable from that of other scarring processes, keloidal histology may be recognized by thickened bundles of hyalinized acellular collagen haphazardly arranged in whorls and nodules with an increased amount of hyaluronidase.
Atrophic scars , on the other hand, are dermal depressions that result from an acute inflammatory process affecting the skin, such as cystic acne or varicella. The inflammation associated with atrophic scars leads to collagen destruction with dermal atrophy. Surgery or other forms of skin trauma may also result in atrophic scars, which are initially erythematous and become increasingly hypopigmented and fibrotic over time. Based on their width, depth, and 3-dimensional architecture, acne scars are sometimes further subclassified into icepick, rolling, and boxcar scars.
Prescars are early wounds in scar-prone skin. Prophylactic or early laser treatment of traumatized skin concomitant with or shortly after cutaneous wounding has been shown to reduce or even prevent scar formation in patients at high risk for scarring. Laser therapy may improve the appearance of wounded skin by promoting better collagen organization in healing wounds.
Laser scar revision
Hypertrophic Scars and Keloids
- •
PDL is the laser of choice for hypertrophic scars and keloids, though the mechanism by which it works is yet to be fully elucidated
- •
PDL may be used alone or in combination with other scar treatments
- •
PDL is relatively safe, but a series of treatments is often necessary
- •
The most common adverse events of PDL irradiation are transient purpura and hyperpigmentation
Detailed reviews of laser scar revision have previously been published. Early in vitro experimentation with a 1064-nm neodymium:yttrium-aluminum-garnet (Nd:YAG) laser demonstrated that fibroblasts irradiated with this wavelength produced decreased amounts of collagen. The Nd:YAG, argon, and carbon dioxide (CO 2 ) lasers were subsequently studied on hypertrophic scars and keloids with early promising results, but high recurrence rates were observed. It was not until PDLs were studied that it was shown that scar size, erythema, pliability, pruritus, and texture could be improved. A wealth of published clinical data over 2 decades has shown that PDL is effective for all forms of hypertrophic scarring and keloids, regardless of etiology. Burn scars, sternotomy scars, acne scars, and facial scars resulting from cutaneous surgery all appear to respond well to PDL. As a consequence of this research, the laser of choice in treating hypertrophic scars and keloids is the vascular-specific 585-nm PDL ( Fig. 1 ).
There is no consensus on the precise mechanism whereby the PDL exerts its effect on scars. The PDL has been demonstrated to reduce expression of transforming growth factor β, fibroblast proliferation, and collagen type III deposition. Other plausible explanations include selective photothermolysis of vasculature, released mast cell constituents (such as histamine and interleukins) that could affect collagen metabolism, and the heating of collagen fibers and breaking of disulfide bonds with subsequent collagen realignment.
Scar revision with the PDL is typically performed on an outpatient basis without anesthesia. All persons present in the room must wear protective eyewear capable of filtering light of 585 to 595 nm to avoid retinal damage.
- •
If topical anesthesia is desired, a lidocaine-containing cream or gel can be applied to the treatment areas 30 to 60 minutes before laser irradiation.
- •
To avoid interference with laser penetration, the skin should be cleansed with soap and water to remove residual makeup, powder, or creams. Flammable solutions, such as alcohol, should be avoided in skin preparation.
- •
Wet gauze may be used to protect hair-bearing areas during treatment and to avoid unnecessary thermal injury to nontargeted skin.
- •
The patient and other individuals present in the treatment room must wear protective eyewear capable of filtering light of 585 to 595 nm to avoid retinal damage.
- •
The fluences chosen are determined by the skin phototype of the patient, the type of scar, and previous treatments applied to the area.
- •
It is prudent to begin treatments with the lowest effective energy densities, using increased fluences on subsequent visits only when the response to the previous treatment is suboptimal.
- •
In general, hypertrophic scars and keloids are treated with moderately low energy densities ranging from 6.0 to 7.5 J/cm 2 when using a spot size of 5 or 7 mm and 4.5 to 5.5 J/cm 2 when using a 10-mm spot size.
- •
Energy densities should be lowered by at least 0.5 J/cm 2 in patients with darker skin and for scars in more delicate or thin-skinned locations (eg, eyelids, neck, chest).
- •
Pulse durations ranging from 0.45 to 1.5 milliseconds are commonly used.
- •
Treat the entire surface of the scar with adjacent, nonoverlapping laser pulses.
- •
The appearance of most hypertrophic scars will improve by approximately 50% after 2 treatments with the PDL using the aforementioned laser parameters.
- •
Keloids often require additional treatment sessions to achieve significant improvement, but some may prove altogether unresponsive.
- •
Laser treatments are typically repeated at 6- to 8-week time intervals.
Any concern regarding patient response to treatment should prompt a test spot or patch in a small area before irradiation of the entire lesion. If postoperative crusting or vesiculation is observed, the fluence applied on subsequent visits should be decreased and retreatment postponed until the skin has completely healed. The fluence and pulse duration can be adjusted if scar proliferation continues despite laser irradiation. Generally speaking, higher fluences and shorter pulse durations result in improved scar size and pliability. However, more aggressive laser settings must be carefully considered in patients with darker skin and for scars in more delicate or thin-skinned locations (eg, eyelids, neck, chest).
The use of concomitant intralesional corticosteroids or 5-fluorouracil has been shown to provide additional benefit in proliferative scars. Intralesional injections of corticosteroids (20 mg/mL triamcinolone) are more easily delivered immediately after (rather than before) PDL irradiation because the laser-irradiated scar becomes edematous (making needle penetration easier). An additional consideration is that when steroid injection is performed before laser irradiation the skin blanches, rendering the skin a potentially less amenable target for vascular-specific irradiation.
The most common side effect of treatment with the PDL is postoperative purpura, which often persists for several days. Pulse durations shorter than 6 milliseconds are almost certain to bruise the skin. Edema of treated skin may also occur, but usually subsides within 48 hours. A topical healing ointment under a nonstick bandage can be applied for the first few postoperative days to protect the skin. Treated areas should be gently cleansed daily with water and mild soap. Strict sun avoidance and photoprotection should be advocated between treatment sessions to reduce the risk of pigment alteration. Hyperpigmentation has been reported with varying frequencies (1%–24%). If skin darkening occurs, further laser treatment should be suspended until resolution of the dyspigmentation has occurred in order to reduce the risk of cutaneous melanin interference with laser energy penetration. Topical bleaching agents (such as hydroquinone or kojic acid) may be applied to hasten pigment resolution.
Although no studies regarding the use of 532-nm potassium titanyl phosphate (KTP) lasers have been published, some practitioners advocate their use for erythematous scars because of their ability to reduce erythema. Similarly, intense pulsed light systems have been demonstrated to improve scar erythema. The 532-nm frequency-doubled Q-switched Nd:YAG may be used to treat pigmented hypertrophic scars. Vaporization of keloid scars by CO 2 laser irradiation almost universally results in scar recurrences, but there is growing evidence that fractional lasers may improve hypertrophic scarring (see later discussions).
Atrophic Scars
- •
Fully ablative and fractional laser systems are the systems of choice to improve atrophic scars
- •
The choice of laser is primarily determined by the severity of scarring and the patient’s ability to tolerate postoperative recovery
- •
It appears that ablative and nonablative fractional lasers produce similar clinical results to ablative lasers with significantly fewer adverse effects
- •
The deeper penetration of ablative fractional lasers may lead to enhanced clinical improvement of scars relative to fully ablative and nonablative fractional systems
Ablative lasers
Successful recontouring of atrophic scars has been achieved with CO 2 or erbium:yttrium-aluminum-garnet (Er:YAG or erbium) laser vaporization. Although other treatments such as dermabrasion and injection of various filler materials can also be used for atrophic scars, their operator-dependent efficacy and side-effect profile, as well as temporary clinical effect (in the case of most filler injections), limit their usefulness and widespread acceptance for the longer term. What popularized laser skin resurfacing treatment for atrophic scar revision was its ability to selectively and reproducibly vaporize skin with improved operator control and clinical efficacy. Clinical and histologic comparisons with dermabrasion and chemical peels showed that a predictable amount of skin vaporization and residual thermal damage could only be achieved through lasers, thereby demonstrating the superiority of laser treatment for skin resurfacing.
CO 2 and Er:YAG lasers work to selectively heat and vaporize superficial skin by emitting energy that is absorbed by intracellular tissue water. Cutaneous laser resurfacing produces an additional skin-tightening benefit through controlled heating of dermal collagen. The depth of ablation correlates directly with the number of passes performed, and usually is confined to the epidermis and upper papillary dermis; however, stacking of laser pulses by treating an area with multiple passes in rapid succession or by using a high overlap setting on a scanning device can lead to excessive thermal injury with subsequent increased risk of scarring. An ablative plateau is reached with less effective tissue ablation and accumulation of thermal injury due to reduced tissue water content after initial desiccation. The avoidance of pulse stacking and incomplete removal of partially desiccated tissue is paramount to prevention of excessive thermal accumulation with any laser system. Persistent collagen shrinkage and dermal remodeling are responsible for much of the continued clinical benefits observed after ablative laser resurfacing. The photothermal effect of ablative lasers on the skin account for shrinkage of collagen and noticeable clinical skin tightening, as well as neocollagenesis and collagen remodeling that leads to marked reduction of skin textural irregularities.
Laser treatment of atrophic scars is aimed at reducing the depth of the scar borders and stimulating neocollagenesis to fill in the depressions. Although spot (or local) vaporization of isolated scars is a viable treatment option, extended treatment (at least an entire cosmetic unit) is recommended for more widely distributed defects to avoid obvious lines of demarcation between treated and untreated sites. In addition, treatment of a larger surface area increases the overall collagen-tightening effect, thereby improving clinical response by making scars appear shallower.
Absolute contraindications to ablative laser skin resurfacing include an active cutaneous bacterial, viral, or fungal infection. Patients with an inflammatory skin condition (eg, psoriasis, eczema) involving the skin areas to be treated should be avoided. Isotretinoin use within the preceding 6-month period and a history of keloids are also considered contraindications to ablative laser treatment because of the unpredictable tissue-healing response and greater risk for scarring. Ablative laser scar revision is typically performed on an outpatient basis and requires a thoughtful approach by both doctor and patient, including thorough preoperative counseling related to the postoperative recovery period. All persons in the room must be wearing protective eyewear. If patients are wearing protective contact lens shields, sandblasted metal ones must be chosen because plastic shields do not meet safety standards for ocular protection during periocular laser irradiation. The concave surface of the shields should be liberally lubricated with an ophthalmic ointment, and care must be taken while inserting and removing the shields so as to prevent corneal abrasions.
- •
The ideal patient for ablative laser skin resurfacing has a fair complexion (skin phototype I or II), although darker skin tones may also be treated.
- •
Various anesthetic options can be employed, including topical, intralesional, intravenous, and general anesthesia.
- •
In general, larger treatment areas (eg, full face) require the use of intravenous or general anesthesia for maximal patient comfort.
- •
Prophylactic antibiotics may be used for full-face procedures or large treatment areas.
- •
When choosing treatment parameters, the surgeon must consider factors such as the anatomic location to be resurfaced, the skin phototype of the patient, and previous treatments delivered to the area.
- •
The requisite protective eyewear and other safety precautions (eg, smoke evacuator to capture laser plume) should be used.
- •
The CO 2 laser is generally used at fluences of 250 to 350 mJ to ablate the epidermis in a single pass.
- •
Short-pulsed Er:YAG lasers that are operated at 5 to 15 J/cm 2 often require several passes to result in a similar depth of penetration as CO 2 , whereas longer-pulsed Er:YAG systems can be operated at higher fluences (22.5 J/cm 2 ) to achieve comparable results in a single pass.
- •
Areas with thinner skin (eg, periorbital) require fewer laser passes and nonfacial (eg, neck, chest) laser resurfacing should be avoided, due to the relative paucity of pilosebaceous units in these areas.
- •
Because of their depth and fibrotic nature, most atrophic scars will require at least 2 laser passes regardless of the laser system chosen for treatment.
- •
It is important that any partially desiccated tissue be removed with saline-soaked or water-soaked gauze between laser passes for char formation to be avoided.
- •
Patients should be seen in-office within 24 to 48 hours then at weekly intervals for 1 month for close monitoring of adverse events.
Immediately after ablation the vaporized skin appears erythematous and edematous, with copious serous discharge and generalized worsening of the skin’s appearance over the first few days. It is imperative that patients be monitored closely for appropriate healing responses and potential complications, such as dermatitis or infection, during the 7- to 10-day reepithelialization process. Full-face procedures or large treatment areas often necessitate the use of prophylactic antibiotics and/or antiviral medications to reduce the risk of infection. The use of topical antibiotics is avoided because of the potential development of contact dermatitis. Application of topical ointments, semiocclusive dressings, and/or cooling masks promote healing and reduce swelling.
Postoperative erythema typically lasts several weeks after ablative laser treatment, due to tissue necrosis. Hyperpigmentation is transient and generally appears 3 to 4 weeks after treatment. Its resolution can be hastened with the use of topical bleaching agents. Although hyperpigmentation is relatively common (particularly in patients with darker skin tones), hypopigmentation is rare. The most severe complications of ablative skin resurfacing include hypertrophic scarring and ectropion formation, both related to overly aggressive laser techniques and/or undiagnosed/untreated suprainfections. Hypertrophic burn scars can be effectively treated with the PDL as previously described, whereas ectropion typically requires surgical reconstruction. Retreatment after ablative laser skin resurfacing should be postponed for at least 1 year to accurately gauge clinical improvement and permit full tissue recovery.
Nonablative lasers
As a consequence of the side effects and prolonged postoperative recovery associated with ablative laser treatment, nonablative lasers were subsequently developed to provide a noninvasive option for atrophic scar revision. The most popular and widely used of these nonablative systems include the 1320-nm Nd:YAG, 1450-nm diode, and 1064-nm Nd:YAG lasers. These devices deliver concomitant epidermal surface cooling with deeply penetrating infrared wavelengths that target tissue water and stimulate collagen production via controlled dermal heating without epidermal disruption. A series of 3 to 5 treatments are typically performed on a monthly basis, with optimal clinical efficacy appreciated several (3–6) months after the final laser treatment session. Sustained clinical improvement of scars by 40% to 50% has been observed after the series of treatments. The low side-effect profile of these nonablative systems (limited to local erythema and edema and, rarely, vesiculation or herpes simplex reactivation) compensates for their reduced clinical efficacy (relative to ablative lasers).
Fractional lasers
Due to a need for more noticeable clinical improvement than the aforementioned nonablative systems, fractional photothermolysis was developed. In its relatively short history, fractional laser technology has progressed rapidly, with nearly 30 commercially available fractional systems on the market. These laser systems may best be classified into two categories: (1) nonablative fractional lasers (NAFL) and (2) ablative fractional lasers (AFL).
The initial fractional laser (Fraxel; Reliant Technologies, Mountain View, CA, USA) involved the use of a mid-infrared (1550 nm) wavelength erbium-doped fiber laser to create microscopic noncontiguous columns of thermal injury in the dermis (referred to as microscopic thermal zones or MTZs) surrounded by zones of viable tissue. The spatially precise columns of thermal injury produce localized epidermal necrosis and collagen denaturation at 125 or 250 MTZ/cm 2 . Because the tissue surrounding each MTZ is intact, residual epidermal and dermal cells contribute to rapid healing. Maintenance of the stratum corneum ensures continued epidermal barrier function.
Histologic evaluation of the MTZ demonstrates homogenization of the dermal matrix and the presence of epidermal necrotic debris, representing the extrusion of damaged epidermal keratinocytes by viable keratinocytes at the lateral margin of the MTZ. The necrotic debris exfoliates over the next several days, producing a bronzed appearance to the skin. The wound-healing response differs from that after ablative laser techniques because the epidermal tissue that is spared between thermal zones contains viable transient amplifying cells capable of rapid re-reepithelialization. Furthermore, because the stratum corneum has low water content, it remains intact immediately after treatment. Therefore, the coagulative wound created by NAFL resurfacing is unique and not simply that of an ablative laser used to make “holes” in the skin. In addition, NAFL resurfacing can provide an advantage over purely nonablative laser treatments, due to the gradual exfoliation of the epidermis with resultant improvement in superficial dyspigmentation. A series of NAFL treatments is required to achieve optimal clinical improvement because only a fraction of the skin is treated during a single session.
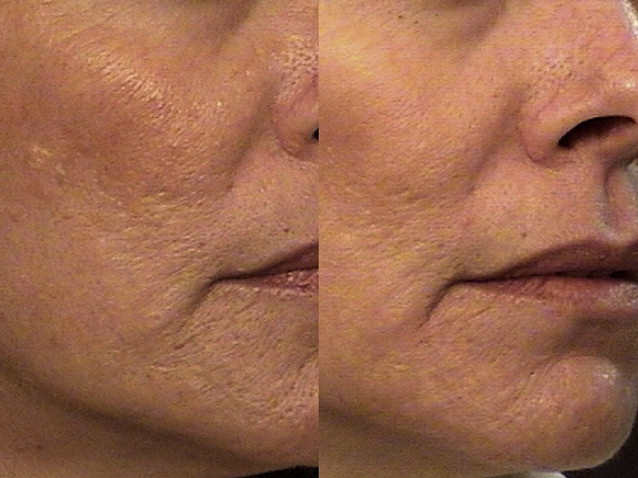
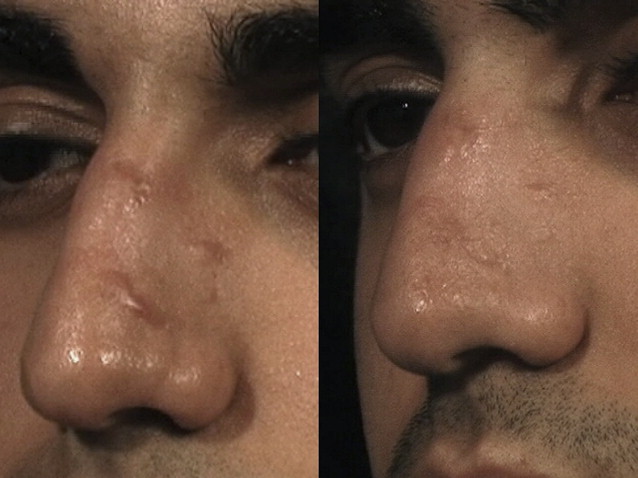
Significant clinical improvement can be obtained when nonablative fractional photothermolysis is applied to atrophic facial acne scars of mild to moderate severity. After a series of 3 consecutive NAFL treatments, clinical improvement of 50% or more is observed in acne scarring ( Fig. 2 ). Similar results have been obtained in scars resulting from other injuries, including surgery and burns ( Fig. 3 ). Patients are treated on a monthly basis, with greater clinical improvement seen with successive treatments. It has been shown that higher energy settings and multiple laser passes translate into improved clinical results while increased density is more likely to result in increased rates and severity of erythema, edema, and hyperpigmentation. By contrast, AFLs (fractionated CO 2 and erbium lasers) not only create similar columns of thermal coagulation through the epidermis and dermis, but also vaporize the stratum corneum. Because of the absence of a protective cap overlying the coagulated columnar regions, the immediate postoperative appearance of treated areas appear more similar to an ablative treatment than that observed with NAFL. Unlike fully ablative treatments, AFLs not only deliver sufficient energy to effect immediate contraction, but intact islands of viable epidermis that facilitate rapid healing remain post treatment. Intense erythema and serosanguinous drainage are evident for 2 to 3 days, followed by complete re-reepithelialization and diminution of erythema by days 6 or 7.