Laparoscopic hand-assisted donor nephrectomy is defined as the removal of either the left or the right kidney from a voluntary kidney donor using minimally invasive laparoscopic techniques assisted by a hand port for the majority of the operation. The kidney is removed from the donor via the hand port and transplanted into the prearranged recipient. The donor and recipient may or may not be related.
Living-donor kidney transplants can only be performed at accredited transplant centers. Accreditation mechanism varies by country. As donors have no medical need for the operation, donor safety is of utmost concern.
A thorough history should be performed. Any history of diabetes, hypercoagulability, hypertension, renal stones, frequent urinary tract infections, abdominal surgery, pre-eclampsia, eclampsia, gestational diabetes, or cardiovascular disease should be explored. A list of absolute and relative contraindications is shown in Table 1. The patient’s family history for these risk factors should also be obtained.
Personal history of cancer or serious infection must be assessed for risk of transmission to the recipient.
Although hypertension is a relative contraindication to donation, selected older donors with well-controlled hypertension on a single antihypertensive medication may be candidates.
Table 1: Absolute and Relative Contraindications
Absolute Contraindications
Relative Contraindications
Diabetes mellitus
Ongoing infection
HIV
Hepatitis B or C
Age <18 y
GFR <80 mL/min
Unsuitable kidney anatomy
Lupus
Sickle cell disease or trait
History of significant cardiovascular disease (e.g., myocardial infarction, stroke, TIA < carotid stenosis, aneurysm)
Pregnancy
History of DVT or PE
Hypercoagulable or hypocoagulable state
Cirrhosis or portal hypertension
Hypertension
Minor cardiovascular disease manifestations
Obstructive or restrictive lung disease
Psychiatric disorders
Substance abuse
Kidney stones
BMI >35
BMI >30 with other risk factors
Proteinuria
Hematuria
Desire to become pregnant in 1-2 y following donation
GFR, glomerular filtration rate; BMI, body mass index; TIA, transient ischemic attack; DVT, deep vein thrombosis; PE, pulmonary embolism.
Obesity is a risk factor for future hypertension, diabetes, and renal insufficiency. Although exact body mass index (BMI) criteria vary by transplant center, typically a BMI less than 30 is preferred, with upper cutoffs between 35 and 40, depending on the center and specific patient history, family history, and habitus. Younger patients with higher BMIs and strong family history of renal risk factors may not be appropriate candidates.
A full physical should be performed, with particular attention for signs of undetected renal disease (e.g., costovertebral angle tenderness) and abdominal scars.
Previous abdominal operations may complicate the laparoscopic approach. Any candidate for a laparoscopic donor nephrectomy must also be a candidate for conversion to an open procedure should that be required.
Potential living kidney donors are also assessed by nephrologist, social worker, and donor advocate. If the potential donor is found to not be a candidate for medical reasons, to be coerced or otherwise inappropriately pressured, or wants to back out, the feedback to the recipient and all others is that the donor is “not an appropriate candidate.” No additional explanation should be given.
Unless in an appropriate protocol, the donor must be ABO-compatible with the recipient. Both the recipient and the donor require two separate blood typing tests. Tissue human leukocyte antigen (HLA)-typing and crossmatching should be performed. If the preliminary crossmatch is negative, the rest of the evaluation may proceed. The crossmatch is repeated a few days before the operation.
For patients who are immunologically incompatible, exchange programs and desensitization protocols may be an option.
Laboratory examination includes electrolytes; creatinine; uric acid; complete blood count; liver function tests; pregnancy testing (in women); prostate specific antigen (in men); HbA1c; and serologies for hepatitis B and C, HIV, cytomegalovirus (CMV), Epstein-Barr virus (EBV), and syphilis. Tuberculosis testing should be performed, along with an electrocardiogram and chest x-ray. Urine testing includes microalbumin and electrolytes, as well as urinalysis and urine culture. Drug testing is performed.
Although glomerular filtration rate (GFR) requirements vary by center and patient history, younger patients should have a GFR greater than 90, whereas older donors (frequently defined as patients >50 years of age) should have a predonation GFR greater than 80. If the GFR is close to the cutoff, an iothalamate GFR nuclear medicine study should be obtained.
Age-appropriate health screening should be up to date. For example, patients older than 50 years of age should have a colonoscopy. Women should undergo age-appropriate mammogram and Pap smear testing. Relatives donating to patients with polycystic kidney disease should be screened for the disease themselves.
Patients with a history of a single kidney stone need a stone evaluation including serum calcium; parathyroid hormone level; and 24-hour urine collection for calcium, oxalate, uric acid, sodium citrate, phosphate, and creatinine. Patients with an extensive nephrolithiasis history are not appropriate living kidney donors.
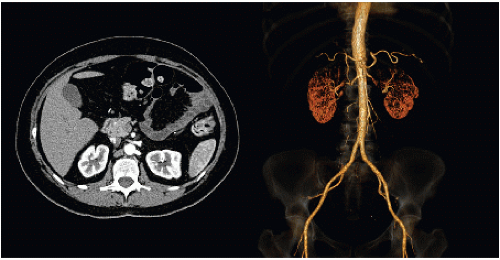
Anatomic evaluation is typically by computed tomography (CT) scan (FIG 1), although magnetic resonance imaging (MRI) can also be used. CT angiogram of the abdomen and pelvis includes a noncontrast phase to evaluate for nephrolithiasis and arterial calcifications, as well as arterial and venous phases, which allow anatomic evaluation for vessel number and anatomy as well as kidney size and ureteral abnormalities. If the kidneys are significantly different in size, a split renal nuclear medicine scan is indicated to determine the percentage of contribution from each kidney.
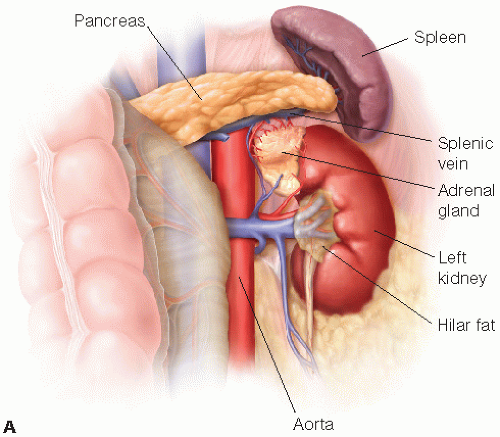
Operative planning is based on the better of the native kidneys remaining with the donor. The left kidney is typically easier to remove, as well as easier to transplant into the recipient, so is often used. Kidneys with multiple vessels are usable, although single artery and vein kidneys are preferred. If there is a kidney stone in one of the kidneys, that kidney typically goes to the transplant recipient. Ex-vivo stone extraction can be done.
Consent of the recipient should include typical operative risks (e.g., hernia, deep vein thrombosis [DVT], damage to nearby structures). In addition, there is a risk of hypertension as well as the remote risk of renal failure for the donor or allograft failure in the recipient.
Laparoscopic living donor nephrectomy is performed in the lateral decubitus position, with the side of the intended donor kidney up and a Foley catheter in place. The kidney rest is used and the bed is flexed to about 30 degrees, with the head kept in line with the spine and kept level.
Care should be taken to place an axillary role, to position the arms appropriately, and to pad the legs. Sequential compression devices (SCDs) and subcutaneous heparin should be used, and the patient should be firmly affixed in place.
Standard skin preparation should be used. Cefazolin is appropriate for preoperative antibiotics.
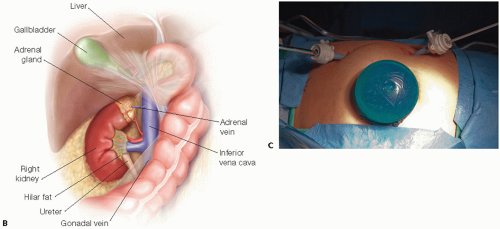
A left laparoscopic hand-assisted donor nephrectomy is described (FIG 2A). The approach to a right kidney is similar, but a perixiphoid 5-mm port is required as well, which is used for placement of a retractor to hold up the liver. In addition to mobilization of the colon, the duodenum must be partially mobilized. Also, the gonadal, adrenal, and lumbar veins typically empty into the inferior vena cava (IVC), instead of the relatively short right renal vein (FIG 2B).
The laparoscopic hand-port incision is made first. The incision is sized for the hand of the primary surgeon, starting just inferior to the umbilicus and extending along the left lateral side of the umbilicus upward on the midline to the appropriate length.
Using the hand to protect the underlying bowel, a bladeless 12-mm port is placed in the left lower quadrant near the edge of the rectus directly lateral to the lower
portion of the midline incision. The hand port is then put in place through the midline incision, and the abdomen is insufflated. A camera port is placed under direct visualization on the same line as the lower port, just under the rib cage (FIG 2C).
The left hand enters through the hand port, the 30-degree camera enters through the upper quadrant port, and the harmonic scalpel enters through the lower quadrant port. The primary surgeon uses the harmonic scalpel and the hand port.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree