Keloids result from an abnormal wound-healing process in which the normal regulatory pathways during tissue regeneration and scar remodeling are disrupted. While the pathogenesis of keloids continues to be investigated, numerous treatment options exist. Although prevention of keloid formation is the best management, early recognition of keloid formation is integral in treatment and prevention of recurrence. Surgical resection with adjuvant silicone gel sheeting or triamcinolone injection is common, but can still result in recurrence. New treatments include chemotherapeutics such as 5-fluorouracil, bleomycin, and mitomycin C. Although further clinical investigation is required for newer treatments, initial results are promising.
- •
Keloids can be difficult to differentiate clinically from hypertrophic scars; however, there are distinguishing characteristics of each
- •
The pathophysiology of keloids continues to require ongoing research
- •
Surgical excision followed by intralesional steroid injection is considered a first line treatment
- •
Silastic gel sheeting can improve keloid appearance when used appropriately
- •
Radiation can be a safe and effective means of keloid treatment with the appropriate precautions
- •
Topical application of chemotherapy medication is a reasonable alternative in patients with keloid recurrence after surgical excision and steroid treatment
Pathophysiology and histology
Wound healing is tightly regulated, and errors in the process can manifest anywhere along the pathologic spectrum from chronic wounds to the aggressive scar formation seen in keloids, the latter being the topic of interest here. On the subject of keloids, hypertrophic scars must also be mentioned because the two are often, incorrectly, used interchangeably. This distinction must be made, as the appropriate application of current and future interventions mandates understanding the clinical, histologic, and biochemical pathology of keloids.
Keloids can occur immediately after trauma, or grow months after a mature, stable scar has formed. This trauma can range from vaccination needle sticks, lacerations, bug bites, and burns, to dermatologic conditions such as acne or folliculitis. In all cases, the end result is skin inflammation. Hypertrophic scars follow the pattern of evolution, stabilization, and involution within the boundaries of the original wound. By contrast, keloids continue to proliferate, resulting in a raised, erythematous scar with a wide variability of height progression and scar distribution. As such, keloids will grow outside the boundaries of the original scar. Although keloids can reach a quiescent phase, very rarely do they regress. Lee and colleagues found that symptomatically, 46% of patients noted keloid-associated pain and 86% noted pruritis.
Keloids affect darker-skinned individuals approximately 15 times more than Caucasians, suggesting a genetic factor. Keloids affect roughly 15% to 20% of the African American and Hispanic populations. Keloids typically occur during and after puberty, between the ages of 10 and 30 years. Although there is no gender predilection, keloids can regress during menopause or worsen during pregnancy.
The pathogenesis of keloids continues to undergo investigation, and understanding this process requires knowledge of the normal wound-healing process. Normal wound healing occurs in 3 stages : the inflammatory phase, the proliferative/granulation phase, and the maturation/remodeling phase. The inflammatory phase begins immediately after the injury. Hemostatic mechanisms of platelet degranulation and activation of complement and clotting cascades occur quickly. Cytokines and growth factors such as transforming growth factor β (TGF-β), platelet derived growth factor (PDGF), and epidermal growth factor (EGF) are released through platelet degranulation and from the surrounding tissue. These cytokines and growth factors induce the influx of neutrophils, macrophages, mast cells, and epithelial cells. After the first 24 to 48 hours, the inflammation is perpetuated by mast cells and neutrophils and can last for anywhere from 3 to 8 days. Macrophages aid in wound debridement as fibroblasts and smooth muscle cells migrate into the wound. Prolongation of this phase occurs in cases of large wounds or in the presence of infection, and results in greater exposure to fibrogenic cytokines.
During the proliferative phase, at approximately 3 to 6 weeks, fibroblasts deposit type III collagen and synthesize granulation tissue, composed of procollagen, elastin, proteoglycans, and hyaluronic acid. This scaffold allows the ingrowth of vasculature, and with wound contracture and closure facilitated by myofibroblasts, allows the wound to undergo continued remodeling in the final phase of wound healing.
The maturation/remodeling phase can take from several months to more than a year. During this phase, the type III collagen is replaced by stronger type I collagen fibers. Proteoglycans are synthesized, and fibrin and fibronectin are degraded. In addition, the extracellular matrix produced by fibroblasts undergoes simultaneous degradation by mostly serine proteases (ie, tissue plasminogen activator and urokinase plasminogen activator) and matrix metalloproteinases (MMPs). Collagen fibers are rearranged, cross-linked, and aligned along tension lines. The tensile strength of the scar improves, but at best achieves only 80% the tensile strength of normal skin.
Histologically, keloids invade the normal surrounding dermis, a distinct difference from hypertrophic scars, which stay within the confines of the wound borders. In keloids, collagen fibers are larger, thicker, wavier, and oriented haphazardly. Collagen fibers in hypertrophic scars and normal scars are oriented parallel to the epidermal surface. The collagen fibers in keloids are arranged into thick collagen bundles that are packed tightly together within the dermis, where there is a lack of sebaceous glands and rete ridges. These collagen bundles are also found to lack the presence of myofibroblasts. Hypertrophic scars form fibrous nodules composed of fibroblasts, mostly type III collagen fibers, and vessels. By contrast, keloid scars form nodules with reduced vascularity and a hypocellular appearance ( Fig. 1 ). This acellular core within keloids is characterized by thick bundles of type I and type III collagen fibers interspersed with fibroblasts (see Fig. 1 B).
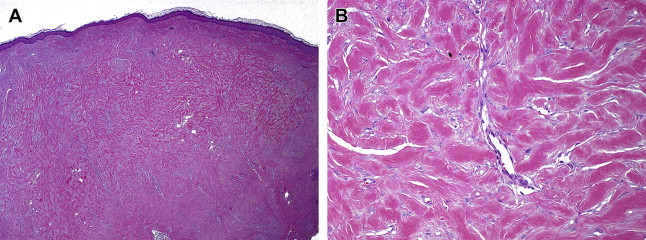
Summarizing the overall histologic findings of keloids, Butler and colleagues classified 4 distinct pathognomonic findings on histology:
- 1.
Keloidal hyalinized collagen
- 2.
Tongue-like advancing edge underneath normal-appearing epidermis and papillary dermis
- 3.
Horizontal cellular fibrous bands in upper reticular dermis
- 4.
Prominent fascia-like fibrous bands.
Despite advances in histologic knowledge of keloids, their exact pathogenesis and etiology remain unclear. Accordingly, the number of possible treatment options reflects multiple hypotheses on how the normal wound-healing process goes awry in keloids. The authors review here the most common theories that are based on accepted observations and proposed contributing factors.
Growth Factors
Elevated levels of TGF-β and PDGF have been found in keloid tissue along with aberrant levels of their activity. The heightened growth-factor activity is likely a result of increased expression of their respective receptors. TGF-β stimulates fibroblasts to produce and deposit collagen and extracellular matrix (ECM) factors. Of interest, keloid fibroblasts exhibit a heightened sensitivity to this growth factor. TGF-β also induces production of PDGF, which controls the rate of granulation tissue formation and stimulates collagen production during the later stages of wound healing. Furthermore, the enzymes that remodel and break down scar ECM are regulated by TGF-β, and decreased levels of collagenase, plasminogen activator, and MMPs could further explain the failure of scar regression evidenced in keloids.
Genetics
Keloids occur more commonly in those of African, Asian, and Hispanic descent, suggesting a genetic susceptibility in darker-skinned individuals. Familial cases of an autosomal dominant pattern of keloids have been reported, and further genetic studies have linked keloid occurrence to loci on chromosomes 2 and 7. Variable gene expression and polygenetic inheritance pattern makes identification of a single gene difficult. In the general population, however, no single gene is responsible for keloid formation.
Dysregulation of apoptosis and altered tumor suppressor gene expression have also been proposed mechanisms of pathogenesis, and studies on p53 and Stat-3 have shown elevated levels compared with normal fibroblasts. Various HLA types have been found to correlate with the keloid phenotype in a Caucasian and Chinese Han population: HLA-DR5, HLA-DQ23, HLA-DQA1, and HLA-DQB1. This finding suggests an abnormal immune response to skin injury in the form of a local immune reaction or possibly a cell-mediated autoimmune reaction.
Skin Trauma
Keloids typically occur after an inciting event, and scar contracture is a necessary step in normal wound healing. Excess mechanical strain on a wound outside of relaxed skin tension lines is a proposed mechanism of keloid formation. Keloids typically occur in younger individuals, a population that naturally possesses greater skin elasticity and tensile strength; this could translate into greater wound tension, mechanical strain, and further influx of hyperfunctioning fibroblasts. Others, however, debate this theory, as some of the most common areas of occurrence are under the least tension (ie, ear, back of the neck). Conversely, the palms and soles of the feet, areas under high tension, are rarely affected.
Given the multitude of contributing factors to the pathophysiology of keloids, treatment options are abundant. Despite numerous options, many investigators agree that the most efficacious route is prevention of keloid formation. In those individuals with a predilection to formation of keloids, caution must be exercised with any procedure that can lead to skin inflammation. Care must be taken to reduce skin tension, wound infection, and foreign-body reaction, with formalized treatment reserved for true keloids and not immature scars.
Treatment options
Steroids
Injection of corticosteroids is frequently employed as first-line therapy for keloids. Although steroids can be used as monotherapy, optimal results have been reported with its use as adjuvant or combination treatment (ie, surgery, cryotherapy, pulse-dye laser, and 5-fluorouracil [5-FU] ). Successful application of steroid injections requires injection into the scar itself, which can be a painful procedure for the patient. Steroids have been shown to induce keloid regression by decreasing collagen and glycosaminoglycan synthesis, reducing the inflammatory process and fibroblast proliferation, and inducing tissue hypoxia through vasoconstriction. In addition, corticosteroids have been found to result in decreased levels of TGF-β and decreased angiogenesis by suppressing endogenous vascular EGF. Side effects can include skin atrophy, depigmentation, telangiectasias, atrophy of subcutaneous tissue and fat, skin necrosis, ulcerations, and Cushingoid features.
Steroid injections as monotherapy have variable efficacy rates ranging from 50% to 100% and recurrence rates from 9% to 50%. Higher success rates can be achieved when treating young, proliferative scars. Several alternative steroid preparations can be employed: hydrocortisone acetate, methylprednisolone acetate, dexamethasone, and triamcinolone acetonide (TA). Although TA is the most frequently employed, there appears to be no benefit derived from using one steroid over another. Numerous therapeutic regimens have been published that commonly tailor dosage and number of treatments based on keloid size and site of the scar. Recommendations vary, from starting dosages of 10 mg/mL to 40 mg/mL of triamcinolone. In the literature, as much as 80 to 120 mg/mL has been used for larger keloids. However, the unifying theme of treatment plans for larger keloids involves frequent scheduled sessions, typically on a monthly basis, for 2 to 3 dosages and may require sporadic treatment for up to 6 months. Because of the pain associated with intralesional injection, many physicians opt to pretreat with lidocaine. The authors prefer to add 1% lidocaine in equal volume to the steroid dose.
With regard to the pediatric population, a great degree of caution must be employed when using corticosteroids. Treatment of children should be monitored closely and have a regularly scheduled follow-up. Various regimens combining TA with other modalities such as 5-FU or pulsed dye laser have been evaluated. Manuskiatti and Fitzpatrick did not find a significant difference in treatment outcome when comparing combination therapy with TA alone. However, these investigators did find faster improvement in keloid appearance using intralesional TA, 5-FU, or both when compared with pulsed dye laser alone. By contrast, Asilian and colleagues showed improved results when combining triamcinolone, 5-FU, and pulsed dye laser.
Despite conflicting data, combination therapy can be a reasonable option in patients who do not respond to single-modality treatment. Nevertheless, TA alone does remain a widely accepted first-line treatment. The publication of international guidelines for scar management in 2002 has further solidified the role of intralesional steroids in the treatment of keloids. This panel further proposed that optimal outcomes are achieved when combining surgical excision and intradermal delivery of corticosteroids.
Compression
Compression therapy has been employed since the 1840s and has been commonly used for prophylaxis after burns since the 1970s. The mechanism of its action has yet to be fully elucidated. However, there are multiple theories :
- 1.
A decrease in blood flow with a resultant decrease in α2-macroglobulin and increase in collagenase-mediated collagen breakdown, normally inhibited by α2-macroglobulin
- 2.
Hypoxia leading to fibroblast degeneration and collagen degradation
- 3.
Lower levels of chondroitin-4-sulfate with increase in collagen degradation
- 4.
Decreased scar hydration, resulting in mast cell stabilization and decrease in neovascularization and matrix production.
Another more recently proposed theory suggests that tissue remodeling is induced by the stimulation of mechanoreceptors. Mechanoreceptors have been found to contribute to cellular apoptosis and overall tissue remodeling by increasing the rigidity of scar extracellular matrix and discouraging the differentiation and proliferation of fibroblasts.
Compression therapy can be difficult for patients to tolerate, as it requires patient adherence to a strict regimen. The goal of compression therapy is to exceed the inherent capillary pressure of 24 mm Hg. Described treatments require the use of compression garments or devices day and night for 6 to 12 months with discontinuation of pressure kept to less than 30 minutes per day. There is a wide variety of commercial materials available for use. A few examples include Coban bandages (3M, St Paul, MN), support bandages and presized garments (Jobst, Toledo, OH), and pressure earrings. While compression therapy has been found to be beneficial, patient compliance can be the limiting factor due to duration of treatment.
Although the use of silicone gel sheeting does not produce much true compression, some investigators do include it in the category of compression therapy. The use of silicone gel sheeting (SGS) is one of the few treatment modalities for keloids that have undergone evaluation in controlled trials. SGS has been found to be highly effective as a prophylactic measure in high-risk individuals, and improves overall scar appearance (ie, elevation, elasticity, redness, and so forth). Other modalities of silicone application include cream containing 20% silicone oil followed by an occlusive dressing that is air-permeable but water-impermeable. More commonly used is a self-drying topical silicone gel that dries to form a flexible, occlusive dressing of silicone, such as Scarguard MD® (Scarguard Labs, Great Neck, NY, USA). Scarguard MD® is a quick-drying liquid containing silicone (12.75%), hydrocortisone (0.55%), and vitamin E, and its use is initiated typically 2 weeks after surgery. Comparative controlled studies have shown that silicone gel is equally as effective as SGS in treating keloids and abnormal scarring. Furthermore, it has been rated by patients as easier to use than SGS.
The efficacy of silicone-based products is likely not attributable to altered oxygen tension, blood flow, or the entry of silicone in scar tissue. One theory suggests altered kinetics of collagenase and resulting scar formation due to an increase in skin surface temperature of approximately 1.7°C (approximately 35°F) while using SGS. One group has proposed that SGS reduces tension at the borders of keloid scars. Another popular theory is that SGS increases tissue hydration by decreasing water evaporation in the stratum corneum. This theory is based on the observation that full-thickness wounds result in injury to the stratum corneum and disruption of the normal fluid homeostasis of the epithelium. The resulting transepidermal water loss can take a year to normalize, and has been found to be higher in cases of excessive scarring when compared with normal skin. This process could lead to increased stimulation of fibroblast collagen production and deposition. Both SGS and silicone gel/cream therapies with occlusive dressing likely restore the epithelial fluid homeostasis to similar degrees. The restoration of the balance of hydration/rehydration by SGS and silicone gel is unique, as similar scar effects have not been found with an occlusive dressing alone.
Surgery
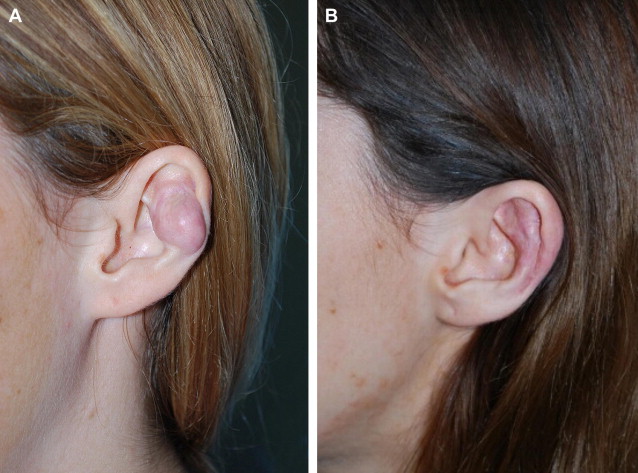
Surgical treatment of keloids can be employed to either reduce keloid mass or radically remove the keloid. Fig. 2 is an example of a left pinna keloid in a 25-year-old Caucasian woman. This patient formed a keloid secondary to ear piercing, and she is currently without recurrence after excision and steroid injections. The reduction of keloid mass without complete extirpation can be used in cases of infection or alleviation of symptoms secondary to keloid mass. Complete excision of keloids is associated with high recurrence rates (50%–100%), and should usually be combined with adjuvant therapies.
The surgeon has the option of anesthetizing the site with a combination of equal parts triamcinolone (40 mg/mL) with lidocaine with epinephrine. If the lesion is pedunculated, an elliptical excision should be performed, followed by undermining of the surrounding skin before closure with sutures. For broader-based sessile lesions, one investigator recommends an alternative closure. Kelly recommends a U-shaped incision when excising large sessile keloids. This U-shaped incision produces a tongue-like flap of keloid tissue. Kelly plans the skin flap to be one-fifth the size of the keloid from its border to the flattest-looking surface. The keloid is excised followed by closure of the site with the tongue-like flap of keloid tissue. Also recommended is suture removal after 10 to 20 days, especially in the case of earlobe keloids.
When operating on a patient with a known history of keloids, care must be taken to follow relaxed skin tension lines. Closure of the defect should be done with maximal wound edge eversion, and minimum tension. Mustoe advocates splinting of the tissue with permanent intradermal sutures (clear nylon), and subcuticular closure with nonabsorbable polypropylene sutures to be left in place for 6 months. However the author does not specify if sutures should be removed or left in situ. Mustoe believes absorbable sutures lose tensile strength too quickly (<1 month) to effectively splint the wound to prevent scar widening or scar hypertrophy. He uses hypoallergenic microporous tape to minimize wound shearing followed by application of SGS as prophylaxis. Mustoe encourages that SGS should be worn for a minimum of 12 hours a day for up to 1 month.
Radiation
Radiation has been used for treatment of keloids since the late 1800s, and there are multiple radiation protocols for keloid treatment, which vary in the type of beam used and the use of radiation as monotherapy or adjuvant therapy. The most common use of radiation is as adjuvant postoperative treatment, although this has not been evaluated in a randomized, prospective controlled trial. Success rates have been recorded in the range of 67% to 98%. Of course there remains some trepidation among physicians regarding the use of radiation to treat a benign disease. Many physicians fear that the use of radiation could produce malignant transformation. In 2009 Ogawa and colleagues conducted a literature search evaluating radiation-associated carcinogenesis between the years 1901 and 2009, and found only 5 reported cases, one of which was a likely malignant transformation of keloid. In the remaining 4 cases the radiation doses and documentation of appropriate protection of surrounding tissue was inconsistent. Leer and colleagues conducted an international survey of radiation oncologists on the safety of radiation on a range of benign diseases, including keloids. Of the 70 institutions in the United States and Canada, more than 90% believed that radiation is a suitable treatment for keloids. In 2007, Leer and colleagues evaluated the use of radiation in the treatment of benign disease throughout the body and classified its use as category A, an accepted indication. Categories B and C were also established, the former recommending use only in clinical trials and the latter not accepted. When using radiation, appropriate protection of thyroid and breast tissue is essential, and its use should be restricted to adults.
The dosage of radiation can be varied. As such, a balance between safety and efficacy should be sought. Recommended dosages range from 10 to 20 Gy with timing of administration within 2 days after surgery. Doses are tailored for the anatomic site, with higher dosages reserved for sites exposed to greater skin tension (ie, chest wall, shoulder, scapula). In 2007 Ogawa and colleagues recommended radiation protocols depending on anatomic site: (1) anterior chest wall, shoulder-scapular region, suprapubic region, 20 Gy in 4 fractions over 4 days; (2) ear lobe, 10 Gy in 2 fractions over 2 days; and (3) for other sites, 15 Gy in 3 fractions over 3 days. Fig. 3 is an example of a radiation mask used during treatment sessions. These masks are used to immobilize the patient, with an area cut out over the target. At the authors’ institution, immobilization masks are used less frequently. Instead, wires are used to isolate the area to be treated and to form a radiation shield that is shaped to the demarcated area ( Figs. 4 and 5 ).


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






