22 Ischemia of the hand
Synopsis
 Upper extremity ischemia is caused by many etiologies and may be classified into acute and chronic cases.
Upper extremity ischemia is caused by many etiologies and may be classified into acute and chronic cases.
 A precise understanding of arterial anatomy is pertinent to preoperative diagnosis, patient selection, and operative procedure.
A precise understanding of arterial anatomy is pertinent to preoperative diagnosis, patient selection, and operative procedure.
 A history of cold intolerance, Raynaud’s phenomenon and frequency of ischemic pain is important. Color changes, ulcerations and infections are evaluated.
A history of cold intolerance, Raynaud’s phenomenon and frequency of ischemic pain is important. Color changes, ulcerations and infections are evaluated.
 Noninvasive vascular examination includes measurement of fingertip temperatures, Doppler ultrasound, segmental arterial pressures and capillaroscopy.
Noninvasive vascular examination includes measurement of fingertip temperatures, Doppler ultrasound, segmental arterial pressures and capillaroscopy.
 Various pharmacologic agents can be used to counteract the sympathetic effect on the muscular layers of the arterial wall in a conservative medical approach.
Various pharmacologic agents can be used to counteract the sympathetic effect on the muscular layers of the arterial wall in a conservative medical approach.
 Surgical intervention is directed at interrupting the sympathetic innervation of the muscle layer, by physical dilatation of the obstructed lumen, or by microsurgical reconstruction of the occluded lumen.
Surgical intervention is directed at interrupting the sympathetic innervation of the muscle layer, by physical dilatation of the obstructed lumen, or by microsurgical reconstruction of the occluded lumen.
Introduction
Hand ischemia occurs when the vascular system is no longer able to transport blood efficiently because of trauma, constriction, obstruction, or vasospasm. It is the critical vascular event that results in eventual tissue necrosis and even amputation without proper management. Compared with the lower extremity, the symptomatology and presentation is varied in the upper limb and it is frequently underdiagnosed. Upper extremity ischemia is caused by many etiologies1 and may be classified into acute and chronic cases (Box 22.1). Any microvascular injury that diminishes the vascular flow induces the acute ischemic conditions of the hand. Systemic, congenital and genetic problems also cause chronic ischemic conditions. Raynaud’s phenomenon is a well-known vasospastic condition that affects 5–10% of the general population.2,3 Primary Raynaud’s disease occurs in the absence of any underlying condition whereas secondary Raynaud’s syndrome is associated with other vasospastic conditions.
BOX 22.1
Etiology of acute and chronic ischemia of the hand
• Atherosclerosis: proximal (more common) or distal
• Intra-arterial drug injection
• Myeloproliferative and immunologic disorder
Reproduced with permission from Jones NF, Emerson ET. Interposition vein graft configurations for microsurgical revascularization of the ischemic hand. Tech Hand Up Extrem Surg. 1999;3:121–130.
Fuchs classified arterial disease according to the three arterial layers: the intima, media, and adventitia. Several of the most frequent pathologic processes (atherosclerosis, intimal hyperplasia) originate in the intimal layer. Endothelial disruption produces thrombotic occlusion and embolization.4 The media consists of smooth muscle cells, fibroblasts and elastic tissue. Atherosclerosis also influences the loss of tissue integrity. The media can become chronically dilated in a diffuse (ectasia) or localized (aneurysm) pattern. The adventitia is involved in diffuse pathologic entities (arteritis, Buerger’s disease). Jones categorized hand ischemia into subgroups of acute and chronic disease. There are multiple causes of upper extremity ischemia (Box 22.1)1 but a classification system based on the underlying pathophysiologic mechanism responsible for producing the ischemia would seem to be most appropriate as a rationale for suitable treatment. Jones described five main pathophysiological mechanisms of hand ischemia: emboli, thrombosis, occlusive disease, vasospasm, and low flow states.5
Regardless of its causes, ischemia of the hand is manifested by color and temperature changes, pale fingers, cold intolerance, numbness, digital ulcerations and gangrenous changes. Despite appropriate treatments, such as smoking cessation, cold avoidance, biofeedback techniques, and pharmacological therapy, ischemia commonly progresses to the eventual consequence of amputation.6–11 This chapter provides an insight to accurate diagnosis and appropriate treatment to avoid devastating tissue losses.
History
Maurice Raynaud described Raynaud’s phenomenon in 1862 and attributed the condition that bears his name to overactivity of the sympathetic nervous system but a review of his cases reveals that most of his patients probably had small-vessel occlusive disease. He hypothesized that “local asphyxia of the extremities’ was a result of ‘increased irritability of the central parts of cord presiding over vascular innervations.12
Lewis first postulated a peripheral rather than central mechanism for Raynaud’s disease caused by a “spasm of digital arteries.” He stated that “the abnormal element in the reaction to cold is a direct reaction and due to a peculiar condition of the vessel wall locally: it is not the result of a reflex through the vasomotor nerves.” However, the pathophysiology remained poorly understood.13
Raynaud’s phenomenon
Raynaud’s disease is a rare disorder but it is an important cause of upper limb ischemia. This entity is often confused with Raynaud syndrome. Raynaud’s phenomenon in patients with upper extremity ischemia is common and classically consists of an orderly progression of color changes (white, blue, and then red) and symptoms in the affected hand secondary to vasospasm. Pallor is followed by a cyanotic appearance, and culminates with reactive hyperemia and burning pain or dysesthesias. This classic pattern occurs in about two-thirds of affected patients. In 1932, Allen and Brown proposed that Raynaud syndrome/phenomenon be differentiated from Raynaud’s disease in that no underlying organic cause can be found in Raynaud’s disease.14 They listed the following criteria as necessary for the diagnosis of Raynaud’s disease:
Merritt,15 and Blunt and Porter16 argued that these criteria are confusing and perhaps obsolete, considering that Raynaud’s phenomenon can be present for an average of 11.5 years prior to diagnosis of an underlying connective tissue disease.17 Furthermore, in one study using sensitive laboratory testing of patients with Raynaud’s disease, more than one-half of patients were found to have some evidence of an underlying systemic disease.18
The relationship between vasospasm in Raynaud’s phenomenon and occlusive disease continues to be controversial. Some researchers have suggested that occlusive disease may initiate vasospasm via humoral and/or sympathetic mediators. This concept is supported by the fact that excision of the occluded arterial segment in patients with traumatic ulnar artery thrombosis (hypothenar hammer syndrome) will improve the digital vasospasm often seen in this disorder.19 Vasospastic symptoms in patients with connective tissue disorders may also be related to an underlying abnormality of the vessel wall. Intimal hyperplasia, thrombosis, fibrosis, embolism, adventitial thickening, aneurysm formation, and calcification have all been found in patients with Raynaud’s phenomenon.20,21 The question remains whether these changes are responsible for the vasospastic symptoms or merely potentiate vasospasm initiated at another site. Therefore, bypass of distal arterial occlusions may not only provide oxygen delivery via increased blood flow to the hand but also may remove a potential source of vasospasm.
Basic science
Anatomy
Embryology
The limb buds appear at 4 weeks of embryonic life as lateral swellings and the forearm vasculature evolves through several stages between 4 and 8 weeks (see Ch. 25). A median artery, ulnar artery and radial artery arise in order from the brachial artery at the elbow, but the median artery involutes as the radial and ulnar arteries provide the majority of blood flow to the hand.
Arterial system in the forearm, hand, and digits
Superficial palmar arch
The superficial palmar arch may be classified as either “complete” or “incomplete.” This classification provides the simplest understanding of the anatomy of the arches.22 Gellman et al. defined an arch to be complete if there was an anastomosis between the vessels constituting it and an incomplete arch as having an absence of communication or anastomosis between the vessels constituting the arch.23 Coleman and Anson also described an incomplete superficial arch as one “in which the contributing arteries do not anastomose, or when the ulnar artery fails to reach the thumb and index finger.”22 Koman et al.24 also reported a complete arch in 78.5% of extremities. Gellman et al. reported a complete arch in 38 of 45 specimens studied (84.4%). In contrast to these studies, Fazan25 reported that only 43% of right hands and 52% of left hands had complete arches.
• Type A: The radioulnar arch is formed by anastomosis between the superficial volar branch of the radial artery and the continuation of the ulnar artery.
• Type B: The superficial arch is formed by a continuation of the ulnar artery and even provides common digital vessels to the thumb and index web space.
• Type C: The median and ulnar arteries combine to form the superficial arch without a contribution from the radial artery.
• Type D: This is characterized by all three vessels (radial, median, ulnar) contributing to the arches.
• Type E: A branch from the deep palmar arch communicates with an ulnar artery initiated superficial arch.
The main branches from the superficial palmar arch are the three common digital arteries to the index-middle, middle-ring, and ring-small finger web spaces22,26 and the proper digital artery to the ulnar border of the small finger.23,27,28 When the princeps pollicis artery and a vessel to the radial side of the index finger (index radial digital artery), originates from the superficial palmar arch, this should be named as the first common digital artery.27,29
Deep palmar arch
The deep palmar arch is less variable than the superficial arch. The radial artery forms the deep arch when it passes from dorsal to palmar by piercing the two heads of the first dorsal interosseous muscle. The arch then curves along the bases of the metacarpal bones. The deep arch may anastomose with one or both volar branches of the ulnar artery.23,30 At least one of the deep volar branches was present in all individuals in a recent study. The deep palmar arterial arch travels across the palm, deep to the flexor tendons, at the level of the carpometacarpal joints to connect with a deep arterial branch from the ulnar artery.22,31 The deep palmar arch gives rise to as many as five palmar metacarpal arteries that pass distally to the level of the metacarpal heads, where they branch dorsally to join the dorsal metacarpal arteries and also connect to the common digital artery through a small palmar arterial branch. Nystrom et al. described three palmar and three dorsal arches which connect the four forearm arteries (ulnar, median, radial, and interosseous arteries). Dorsal metacarpal arteries which originate from the dorsal carpal arches pass to their respective web spaces distally, where they join perforating vessels from the palmar circulation.32
The most consistent of the dorsal vessels supplying the dorsal carpal rete, which consists of numerous small, thin vessels (0.3–0.5 mm), is the radial dorsal carpal branch. It branches off the radial artery 10–15 mm distally to the radial styloid.23
When the superficial arch is well developed, the deep palmar arch is correspondingly less developed and vice versa. A reciprocal inverse relationship was also found between the common palmar digital arteries and the palmar metacarpal arteries.22,30,33,34
Digital arteries
The ulnar digital arteries of the thumb and index finger are larger than the radial digital arteries. The radial digital artery is larger in the ring and small fingers. The difference between the radial and ulnar digital arteries is statistically significant only in the border digits. The anatomy of the thumb digital arteries is unique. The blood supply of the thumb comes mainly from the princeps pollicis artery, the terminal branch of the superficial palmar arch, and the first dorsal metacarpal artery. There are numerous sources of blood supply from the radial and ulnar arteries, including the palmar ulnar, palmar radial, dorsal ulnar and dorsal radial arteries. The first palmar metacarpal artery is absent in about 2% of patients.22,31 This accounts for the tolerance to ischemia by the thumb due to numerous collateral blood vessels.
According to the Hagan–Poiseuille’s law, the blood viscosity, diameter, length and the pressure gradient influence flow through the digital arteries, and larger digital arteries can carry more blood. Strauch and de Moura described the numerous interconnections between the digital arteries beyond the metacarpophalangeal joints. These connections play an important role in hand ischemia if segmental arterial obstruction develops.34
Micro-arterial system
Microvascular vessels are defined as having a diameter of <100 µm. Their role is to deliver oxygen and nutrients at a cellular level. They consist of nutritional capillary and thermoregulatory vessels.33 In the digits, 80–90% of the total flow passes through thermoregulatory beds and 10–20% are involved in capillary nutrition.35 In pathologic states, cellular hypoperfusion leads to ischemic symptoms and imbalance in the distribution of thermoregulatory and nutritional flow resulting in cell death or damage.
Physiology of blood flow
Hemodynamics
Koman et al.24 described macrovascular structures that are defined as vessels >100 µm. Their function is to deliver nutrients to the microvascular beds, provide adequate capacity for arteriovenous thermoregulatory flow and drain nutritional and thermoregulatory beds.
Cellular control mechanisms
Blood flow does not only follow principles of fluid dynamics. There is compensation between arterial dilatation, collateral vessels, and resistance in the peripheral circulation. In a normal extremity, blood flow in the hand depends on sympathetic tone, metabolic demands, environmental events, local factors, and circulating humoral mediators. Alpha-adrenergic control is the dominant control arm of vasoconstriction, however vasodilatation is initiated by endothelium-derived relaxing factor. Active vasoconstriction and vasodilatation may be initiated by a central control process mediated through peripheral neural structures or circulatory factors or by local autoregulation, which is metabolic or myogenic. Metabolic autoregulation occurs in response to local metabolic needs and is mediated by decreased oxygen and build-up of adenosine and potassium.36 Myogenic autoregulation is mediated via transmural pressure and stretch-operated calcium channels.37 The microcirculation is also affected by endothelial factors within blood vessels. The endothelium plays a major role in control of vasomotor tone and blood fluidity, lipid metabolism and finally angiogenesis. The endothelium has been recognized as an active tissue responsible for elaborating both vasodilatory and vasoconstrictor substances. Endothelial cells respond to differences in intravascular pressure due to variations in the size of the lumen by releasing endothelium-derived relaxing factor.38 Endothelium-derived relaxing factor produces active vasodilatation, and endothelin is a potent vasoconstrictor. Both compounds are released from the endothelial cells to regulate vascular flow. Endothelial cells can also release thromboxane A2, prostacyclin, and the molecule of thrombomodulin and heparin sulfate.
Pathophysiology
As described previously, Fuchs classified arterial disease according to the three arterial layers: intima, media, and adventitia. Several pathologic processes (atherosclerosis, intimal hyperplasia) originate from this intimal layer. Endothelial disruption produces thrombotic occlusion and embolization.4 The media consists of smooth muscle cells, fibroblasts and elastic tissue. Atherosclerosis leads to loss of tissue integrity, and the media becomes chronic dilated in a diffuse (ectasia) or localized (aneurysm) pattern. The adventitia is involved in diffuse pathologic conditions (arteritis, Buerger’s disease).
A classification system based on the underlying pathophysiological mechanism responsible for producing the ischemia would seem to be most appropriate as a rationale for determining treatment. The pathological processes that produce ischemia in any end organ (and the hand can be considered such an end organ) are emboli, “sludging” of blood in low flow states, thrombosis, external compression, intimal proliferation progressing to occlusion (arteriosclerosis), and vasospasm.5
Trauma
Traumatic causes of ischemia can be occupational, iatrogenic, or secondary to an injury. Hypothenar hammer syndrome (HHS) first described by Van Rosen in 1934 is an uncommon cause of secondary Raynaud’s phenomenon, and occurs mainly in patients who use the hypothenar part of their hand as a hammer.39
Because of its anatomic configuration within Guyon’s canal, the ulnar artery is particularly vulnerable to mechanical injury. The hook of the hamate bone presses against the superficial palmar branch of the ulnar artery in Guyon’s canal, leading to the development of progressive periadventitial scarring, damage to the media, disruption of the internal elastic lamina, intimal damage, and subintimal hematoma of the ulnar artery. These events are postulated to be the pathophysiological mechanism whereby repetitive trauma causes thrombosis in competitive athletes (volleyball, karate, handball, and baseball).40–42
‘Vibration white finger’ or vibration-induced Raynaud’s phenomenon is also related to repetitive trauma in workers who frequently use drills, jackhammers and chain saws. High frequency vibration is assumed to affect the response of the sympathetic vasoconstrictor nerves and receptors, not only to mechanical but also to thermal induced pain.43,44
Radial artery catheterization frequently results in radial artery thrombosis, although it is frequently asymptomatic because of communication between the radial and ulnar arterial systems. However, if the patient has an incomplete superficial palmar arch, distal ischemia may occur.23 Cardiac catheterization via the brachial artery may result in thrombosis and distal emboli, with an incidence of approximately 0.6%.45
Arteriovenous shunt operations in renal failure patients may also result in hand ischemia. The arteriovenous shunt may redirect a critical volume of blood flow away from the hand, resulting in a “steal” phenomenon producing severe motor and sensory deficits.46,47
Diagnosis/patient presentation
History and physical examination
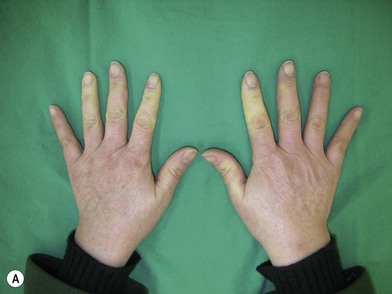
A history of cold intolerance, Raynaud’s phenomenon (Fig. 22.1), and frequency of ischemic pain is important, as is smoking history; occupation involving repetitive injury or vibration trauma, and systemic disease including diabetes, cardiac disease, arrhythmias, drug use, blood dyscrasias and peripheral neurological abnormalities. Unilateral Raynaud’s symptoms are especially suspicious and are usually indicative of occlusive disease on the affected side.
McCabe et al. and Troum et al. developed a questionnaire that can be used to assess the severity of cold sensitivity and quantify the magnitude, duration, and frequency of vasospastic symptoms and their effect on function.48,49
A thorough examination of the upper extremity for previous trauma, old scars or operative incisions, skin color, temperature, presence of digital ulcers, and motor and sensory evaluation of the three peripheral nerves should be routine. Observation of blood flow in the fingernails may help to diagnose ischemia due to connective tissue disease.25,50
Palpation may detect an abnormal mass or thrill. The brachial, radial and ulnar arterial pulses are also palpated. The Allen test allows rapid evaluation of the patency of radial and ulnar arterial inflow into the hand. A digital Allen test can occasionally be performed either by forcing blood out of the finger or by sequential compression of the digital arteries.51,52
Color changes, ulcerations and infections are evaluated. Nonhealing ulcerations and/or impending gangrene associated with unilateral Raynaud’s symptoms are presumptive evidence of thrombosis or embolism.25,53
Diagnostic investigations
Capillaroscopy
The capillaries of the nail fold can be examined directly by specialized dynamic videophotometric capillaroscopy, which provides measurements of capillary diameter, red blood cell velocity and total flow. It can be difficult to perform in 10–12% of individuals due to anomalous vessel orientation, varying length of capillary loops, hyperkeratotic skin or dense pigmentation. Dynamic capillary videomicroscopy allows direct assessment of nutritional perfusion and arteriovenous shunting, and provides objective evidence of the effects of systemic disease on the microcirculation and objective confirmation of any improvement with medical intervention.25
Ultrasound
The pencil Doppler probe can also be used to assess the patency of the distal radial and ulnar arteries at the wrist and the dorsal branch of the radial artery as it passes through the web space, the superficial palmar arch, the common digital arteries and each proper digital artery on the radial and ulnar aspects of each digit. Jones found the pencil Doppler probe to be the simplest, most informative technique providing precise determination of the patency of the arterial structures. Doppler evidence of occlusion of either the distal radial or ulnar arteries is one of the primary criteria for proceeding to invasive arteriography.1,5
Segmental arterial pressures of the brachial artery at the elbow and the distal radial and ulnar arteries at the wrist may be measured with a pencil Doppler probe. A Radial-Brachial Index (RBI) and a Digital-Brachial Index (DBI) can be calculated with a value of ≤0.7 indicating that arterial outflow to the hand is decreased.54
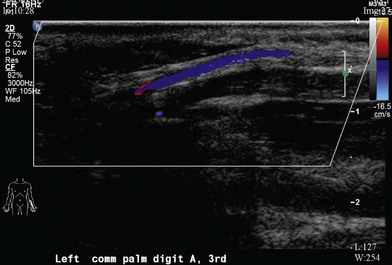
Duplex ultrasonography
A Duplex ultrasonogram is noninvasive, repeatable, and can be used for follow-up investigation. Its most important advantage is presenting real time blood flow information (Fig. 22.2).55,56
Isolated cold stress testing
Cold stress testing was developed to provide an evaluation of the digital response to physiologic stress. Digital temperatures or digital plethysmography (pulse volume recordings) are measured before, during, and after application of a cold stress-immersion in cold water or a cold chamber at 4°C.57 If the fall in digital temperature or drop in pulse volume recording can be partially ameliorated by a prior injection of local anesthetic, this implies that vasospasm is a likely mechanism for the ischemia.
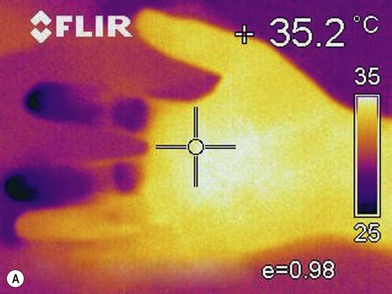
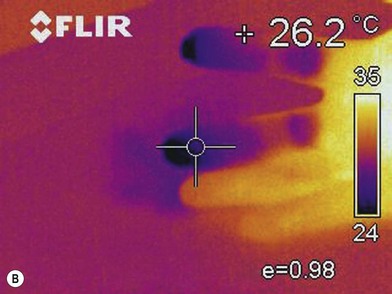
Infrared thermography
Infrared thermography (IRT) provides a measure of the decreased skin temperature of patients with hand ischemia due to decreased superficial skin blood flow.44 The advantage of IRT is that it is noninvasive, easy to use and inexpensive (Fig. 22.3). However, it is influenced by the ambient temperature, and skin temperature itself does not reveal the condition of a blood vessel and cannot visualize the site of stenosis.
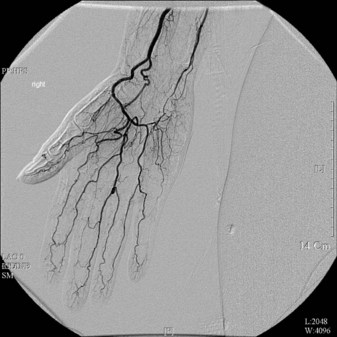
Angiography
Potential disadvantages of conventional angiography include bleeding, allergic response to the contrast media, and X-ray exposure. A major problem with invasive angiography is the vessel spasm induced by the contrast media (Fig. 22.4).23,56,58 This can be circumvented by performing angiography under a brachial plexus block or by preventing vasoconstriction by intra-arterial injection of 4 mg phentolamine.59
Angiography defines the site and extent of thrombosis or occlusion of the distal radial and ulnar arteries and even the common digital and proper digital arteries.23,58 Jones5 has suggested some criteria for angiographic investigation of upper extremity ischemia as follows: (1) Unilateral Raynaud’s phenomenon; (2) progressive digital ulceration or gangrene despite good medical management; (3) recurrent digital ulceration; (4) Doppler evidence of occlusion of a major inflow artery, and (5) acute onset of ischemic symptoms.
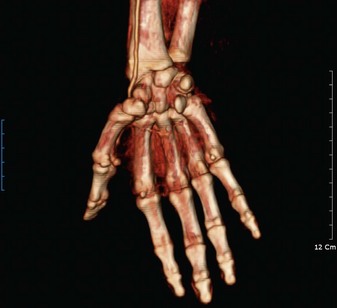
MR and CT angiography
More recently, high-resolution contrast enhanced MR angiography and CT angiography continue to be refined to compete with conventional angiography.60 Even though enhanced MR angiography and CT angiography are noninvasive, the resolution still does not allow the same visualization of the digital arteries compared with conventional angiography. Currently, if the surgeon requires accurate visualization of the digital arteries, conventional angiography is still preferred (Fig. 22.5).
Patient selection
Acute ischemia
Acute arterial injury
Acute ischemia of the hand may occur following laceration of the brachial, radial, and ulnar arteries. Closed vascular injuries are often caused by high energy trauma with accompanying fractures.61 High energy trauma may cause hemorrhage, thrombosis, incomplete vessel injury with development of an aneurysm, compartment syndrome, or progressive thrombosis and distal embolization.62 Specific injuries of the upper extremity that may result in ischemia of the hand include shoulder dislocation, posterior dislocation of the elbow and supracondylar fracture of the humerus. Diagnosis is usually not difficult in patients with significant hemorrhage and distal ischemia. Undetected injury to noncritical vessels may result in a false aneurysm or formation of an arteriovenous fistula.63 Thal described the indications for operative exploration.64
Physical findings of a pulsatile hematoma, a thrill, an audible bruit, decreased peripheral pulses, or associated neurologic deficits are very important, but the presence of a distal pulse is not reliable in the evaluation of vascular integrity (Box 22.2). Immediate diagnosis is crucial in patients with acute ischemia of the hand, and this has been accomplished most expeditiously with Doppler examination and angiography.
Arterial emboli
The heart is the most common origin for arterial emboli. Acute onset of pallor, pain, coolness, paresthesias and pulselessness should lead to strong suspicion for embolism. Ischemia will produce muscle paralysis after an embolus proximal to the forearm muscles, but paralysis is not present in distal emboli. Segmental arterial pressures are helpful, and arteriography of the entire upper extremity will determine if there is a more proximal arterial source of emboli and also will help differentiate embolism from acute arterial thrombosis.70