Intraoperative Radiotherapy
Lydia Choi
Elisa Perego
Virgilio Sacchini
Introduction
Rationale for Intraoperative Radiotherapy
The rationale for the use of segmental radiation therapy in place of whole-breast irradiation is based on the finding that approximately 85% of breast relapses are confined to the same quadrant of the breast as the primary tumor (1). Tumoral foci are usually near the primary tumor, and residual microscopic disease occurring in the same quadrant as the resection is often the cause of local disease recurrence. Phase I and II trials have demonstrated that single-dose intraoperative radiotherapy (IORT) for localized breast cancers can be applied without increasing the normal rate of complications after surgery. Other studies with brachytherapy with longer follow-up demonstrated that partial radiation therapy can be performed safely with good local control and good cosmesis in selected patients.
Advantages of IORT
The ability to deliver a single therapeutic dose of radiation to the tumor bed during surgery or within 5 days after surgery, thereby avoiding the standard 5- to 6-week external-beam treatment, may benefit patients through alleviating psychological distress, allowing earlier return to normal life, and reducing related expenses, including the cost of the procedure and other indirectly associated expenses.
Convenience and Cost
Shortening the course of radiotherapy from the standard 6-week regimen to a single-dose intraoperative technique lowers costs and allows more convenience for patients. It also increases compliance to 100% and eliminates the attrition rate during standard radiotherapy because of side effects or logistical difficulties in traveling to the radiation facility (1). It may allow more breast conservation in rural geographic areas where radiation facilities or transportation are not readily available and where many women must undergo mastectomy because of these secondary issues.
Accuracy
Precisely targeting therapy to the lumpectomy cavity with IORT also directs radiation to the site most likely to need therapy and spares the brachial plexus, heart, and lungs. It avoids an incorrectly directed boost of standard radiotherapy because of lack of clips or seroma to define the lumpectomy cavity accurately. Benda et al. (2) showed discrepancies between radiation oncologists in planning cavity boosts, with variation in targets of more than 1 cm. Radiation to the skin is also reduced by shielding and may be associated with improved cosmesis and possibly elimination of radiation-induced angiosarcoma.
Elimination of Delay in Receiving Radiotherapy
When radiation is delayed because of timing around chemotherapy, there is evidence to suggest increased local failure (3). Although data are conflicting, irradiating immediately renders arguments for or against giving chemotherapy first moot.
Expanded Indications for Radiotherapy
Previously irradiated patients, either for Hodgkin disease or previous cancer, are generally considered to be ineligible for re-irradiation if they have recurrence. IORT has been found to be a promising option for these patients (4).
Partial radiation therapy has been tested in several clinical phase I and II studies and is currently under investigation for phase III in a major nationwide National Surgical Adjuvant Breast and Bowel Project – Radiation Therapy Oncology Group (NSABP-RTOG) study randomizing breast cancer patients submitted to conservative surgery to receive conventional whole-breast external-beam radiation therapy or partial radiation therapy in the form of three-dimensional (3D) conformal external beam, brachytherapy with MammoSite or brachytherapy with iridium implants. Other trials in Europe such as the Electron Intraoperative radioTherapy (ELIOT) trial and TARGeted Intraoperative radioTherapy (TARGIT) trial are studying the delivery of partial radiation therapy during surgery, immediately after quadrantectomy, or lumpectomy.
As part of the TARGIT trial, criteria for IORT are defined as age greater than 40 years and unicentric invasive breast cancer less than 3 cm in size treatable by lumpectomy (5). The European Institute of Oncology trial limits participants to patients older than 48 years with unifocal small invasive cancer (maximum tumor diameter 2.5 cm).
Because IORT is still considered an experimental treatment for breast cancer, it has been limited outside of trials in use to older patients with early-stage cancer or patients who are not candidates for standard whole-breast irradiation, such as those previously irradiated or those with severe comorbidities (6). It has also been evaluated as a boost dose with standard radiotherapy to the whole breast, although when this is decided on as the course of treatment, a longer interval of 5 to 6 weeks between IORT and standard radiotherapy seems to be associated with reduced morbidity compared with intervals shorter than 4 weeks (7). Another use of IORT is as a dose to the nipple–areolar complex after nipple-sparing mastectomy. A total of 800 patients receiving IORT were compared with 200 receiving standard radiotherapy to the nipple–areolar complex with no significant difference in outcome, even when a group with close margins was evaluated (8).
Patients with bilateral breast cancer, multifocal or multicentric breast cancer, clinically positive lymph node metastasis, or extensive ductal carcinoma in situ (DCIS) are not candidates for IORT. Exclusion criteria for standard radiation also apply, such as collagen vascular disease and pregnancy, although a preliminary dose analysis of potential fetal radiation exposure in Milan showed negligible delivery to the fetus (9).
Relevant Surgical Anatomy
The boundaries of the breast include the clavicle superiorly, the sternum medially, and the latissimus dorsi posterolaterally. When brachytherapy is used, the lumpectomy is performed in a standard fashion, either with or without preoperative needle localization. In the United States this usually involves removal of less tissue than in Italy (the home of the ELIOT IORT trial) where quadrantectomy with removal of skin, parenchyma, and muscle fascia is often the norm. At least 1 cm of tissue from the tumor bed to either the skin or chest wall should remain after lumpectomy for the best cosmetic result from IORT.
There are two main methods of delivering IORT: electron beam through linear accelerators and brachytherapy. Several different machines are in use for delivering electron beams, and whereas previously these were stationary, currently most are portable. Mobile linear accelerators include Linac (Info&Tech, Rome, Italy), Novac7 (Hitesys Srl, Aprilia, Italy), Mobetron (IntraOp Medical Corp, Santa Clara, California), and Intrabeam (Zeiss, Inc, Oberkochen, Germany). These machines are portable electron delivery systems that can be moved into the operating room. Electron beams are delivered at variable energies (3, 5, 7, and 9 eV) with maximum energy of 10 to 12 MeV to limit possible exposure to other operating rooms (10).
The Intrabeam, which is the system used in the TARGIT trial, deserves mention for variation from this description. It is a photon radiosurgery system that has been approved by the Federal Drug Administration for use in any part of the body since 1999 (11). Photon radiosurgery also uses electron beams, but these are used to generate X-ray photons at the tip of a wand-like instrument that is inserted into the lumpectomy cavity. This results in delivery of radiation from the lumpectomy cavity outward, as with brachytherapy. The limit of this method is the superficial penetration of the radiation beam: only 2 mm of tissue surrounding the lumpectomy cavity receives the therapeutic dose of 20 Gy.
Procedure Steps
The method of IORT delivery determines the extent of breast dissection.
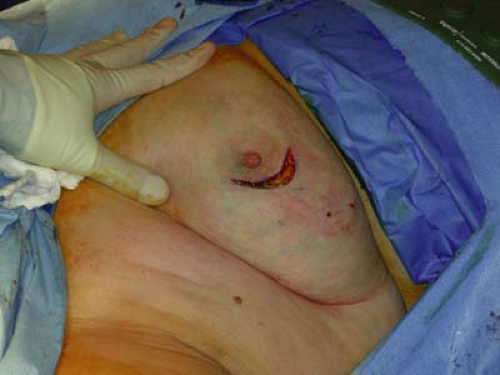
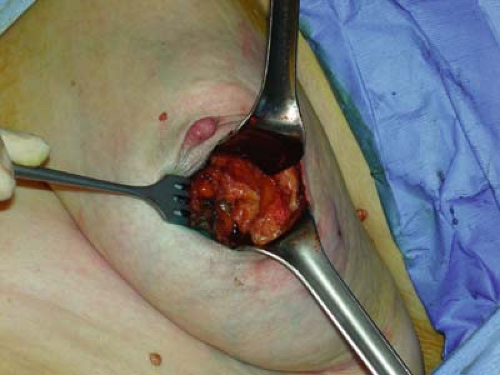
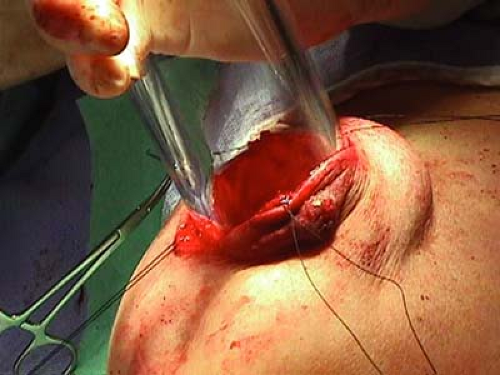
For all mobile linear accelerators, the lumpectomy or quadrantectomy is initially performed as usual. A skin incision is made, then dissection carried through subcutaneous fat and breast parenchyma to reach the tumor, which is removed with a 1 to 2 cm margin of normal surrounding tissue (Figs. 17.1 and 17.2).
Skin and breast parenchyma must then be mobilized for protection during IORT delivery:
Skin flaps are raised from the underlying breast tissue for a few centimeters circumferentially to allow retraction away from the radiation source.
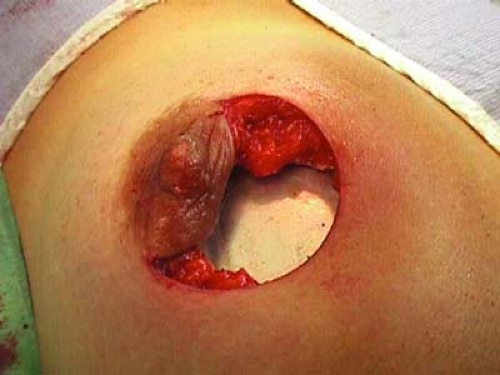
Breast parenchyma is then lifted free from the pectoralis muscle to allow insertion of a protective aluminum-lead disk over the pectoralis (Fig. 17.3).
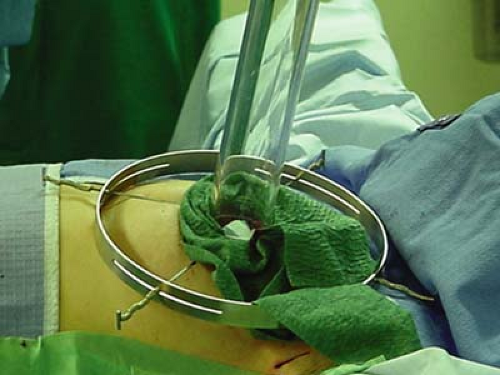
Radiation beams are collimated, or aligned, by a 5-mm-thick Perspex tube that has two parts: the sterile portion is placed in the operative field by the surgeon before connection to the distal portion, which is managed by the radiation oncologist. Electron beams are delivered perpendicular to the tissue for 2 minutes, with electron energies corresponding to the distance of penetration (Fig. 17.6).
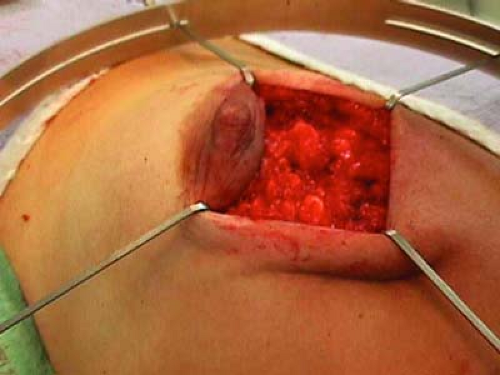
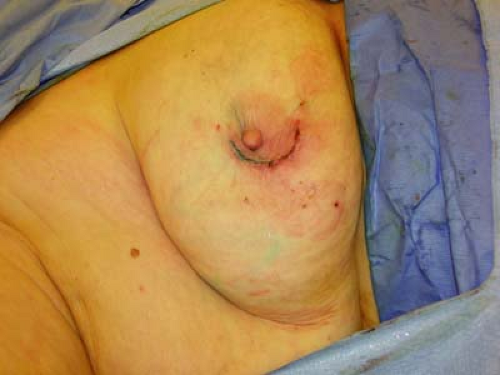
After radiation delivery, the tube is removed and the lumpectomy incision closed as usual, with or without breast parenchymal closure (Fig. 17.7).
The other method of delivering IORT is brachytherapy. Radiation is delivered at high-dose rate through remote afterloading—in other words, a relatively high dose of
radiation (20 Gy) is temporarily delivered through tubing connected to a Silastic template, which is inserted into the lumpectomy cavity. The main difference between this technique and the electron beam therapy is that the skin and chest wall do not have to be protected from the radiotherapy source because radiation is delivered internally to externally and diffuses sufficiently by the time it reaches the skin and muscle, thus avoiding significant toxicity (12).
radiation (20 Gy) is temporarily delivered through tubing connected to a Silastic template, which is inserted into the lumpectomy cavity. The main difference between this technique and the electron beam therapy is that the skin and chest wall do not have to be protected from the radiotherapy source because radiation is delivered internally to externally and diffuses sufficiently by the time it reaches the skin and muscle, thus avoiding significant toxicity (12).
With this method, the lumpectomy is again performed as usual. A 1 cm margin of normal breast tissue between the radiation source, skin, and chest wall is preferred. The skin is retracted using the Lone Star Retraction System (Fig. 17.8).
The sterile applicator is inserted into the lumpectomy cavity and the tubing connected to the radiation delivery source. The method used at our institution is to size the cavity with phantom Harrison–Anderson–Mick (HAM) applicators (Figs. 17.9 and 17.10). When the correct size is found, a corresponding real HAM applicator is inserted into the cavity (Fig. 17.11).
The skin edges are covered with sponges or towels to protect them from the radiation source (Fig. 17.12).
Computer-calculated dosimetry is used to determine the dwell time of the radiation source within the catheters and the total treatment time (Fig. 17.13).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree