Fig. 41.1
The donor face and scalp flap was marked preoperatively using marking pen. (a) Dorsal view. (b) Ventral view. (c) Side view. (d) The harvested total composite face/scalp flap. (e) The donor rat after total composite face/scalp flap harvest. The donor rats were sacrificed after flap harvesting
Operative Technique for the Recipient
An antibiotic (potassium penicillin, 100,000 international units/kg, IM, bid) was prophylactically administered preoperatively. Lactated Ringer’s solution (5 ml, SC, every 4 h) was administered as a fluid supplement during the operation and on postoperative days 0 and 1.
A 1.5 cm wide skin excision was planned at the back of the recipient. A defect was created in the back of the recipient rat by excision of the 1.5 cm skin bridge. In groups I and II the harvested flap was placed to the defect area as a graft with no microvascular anastomosis (Fig. 41.2a–c). In group III the rat was turned to the prone position and the femoral arteries and veins were prepared. Following the preparation of the recipient’s vessels microsurgical anastomoses were performed bilaterally between the carotid arteries and the femoral arteries, and between the jugular veins and the femoral veins at the groin region (Fig. 41.3a–c).
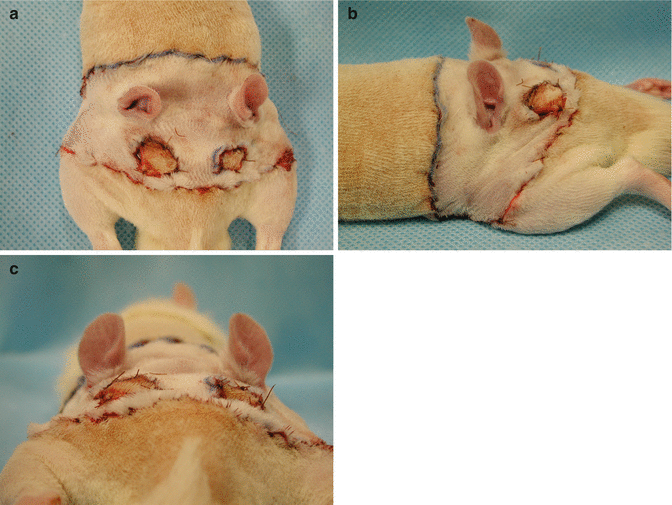
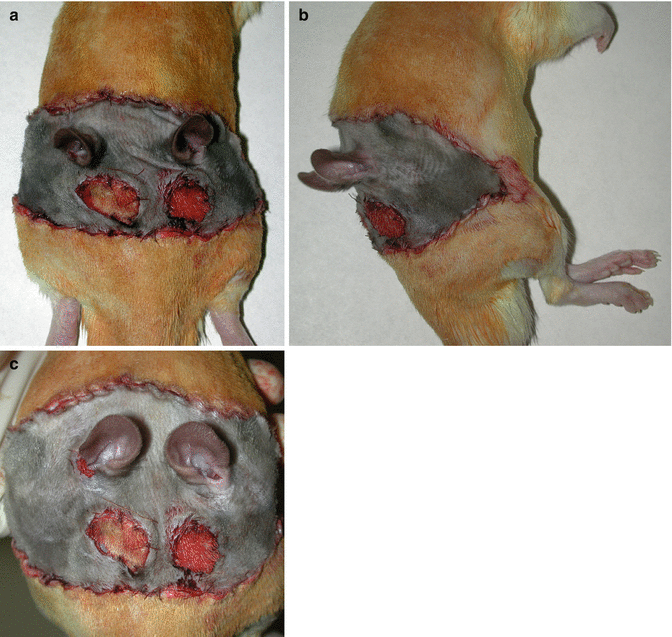

Fig. 41.2
In group I the harvested isogeneic flap was placed to the defect area as a graft with no microvascular anastomosis. (a) Dorsal view. (b) Side view. (c) Caudal view

Fig. 41.3
In group III the harvested allogeneic flap was placed to the defect area and carotid artery and femoral artery, jugular vein and femoral vein anastomoses were performed using microsurgical techniques. (a) Dorsal view. (b) Side view. (c) Caudal view
Postoperatively, all animals were caged individually under standard environmental conditions. They were maintained on commercially available balanced rodent food, which was available ad libitum. Postoperative analgesia was provided with buprenorphine (0.5 mg/kg, administered subcutaneously every 12 h) for the first 2 days.
Immunosuppressive Protocol
To prevent acute and chronic allograft rejection, cyclosporine-A (16 mg/kg per day) therapy was initiated 24 h after transplantation in the allogeneic transplantations groups. The dose was tapered to 2 mg/kg per day after 4 weeks and was maintained at that level thereafter.
Results
A total of 16 composite face/scalp graft and flap heterotopic transplantations were performed between Lewis and Lewis rats and between LBN and Lewis rats. The procedure required an average of 4 h, and the ischemia about 1 h.
In the control groups with non-vascularized transplants (group I and II), flap necrosis within 5–7 days after transplantation was observed in all transplants (Fig. 41.4a, b). Animals were then sacrificed by intracardiac lidocaine injection. No other complications were observed in group I and II.
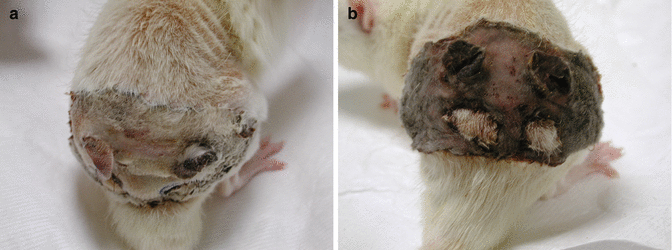

Fig. 41.4
In the control groups with non-vascularized transplants (group I and II), flap necrosis within 5–7 days after transplantation was observed in all transplants. (a) Total necrosis of the transplanted isogeneic nonvascular graft in group I. (b) Total necrosis of the transplanted allogeneic nonvascular graft in group II
The common carotid arteries and external jugular veins of the donor were used for all transplantation procedures in group III. Successful transplantations, with minor complications and the highest animal survival rate, were achieved by using carotid artery and femoral artery anastomoses bilaterally and jugular vein and femoral vein anastomoses bilaterally.
During the first 3 days after transplantation, the animals were closely monitored. Special attention was paid to analgesia and adequate fluid supplementation, to facilitate recovery. On post-transplantation day 5, the animals returned to their normal routine of eating, drinking, and playing. Mild soft-tissue edema of the flaps were observed which was spontaneously resolved within 3 weeks after transplantation.
Two allograft recipients died at the early postoperative period in group III, they were not replaced. The other allograft recipients (n = 2) from group III survived more than 400 days with low dose Cyclosporine-A therapy. There were perfect flap viability without signs of infection, rejection, or illness attributable to the Cyclosporine-A therapy (Fig. 41.5a, b). The recipients were sacrificed at day 450 postoperative.
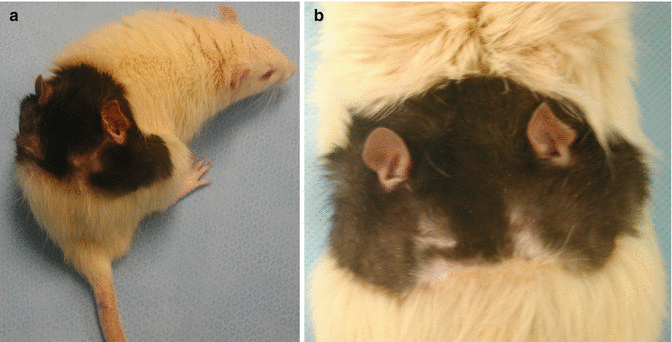

Fig. 41.5
There were perfect flap viability without signs of infection, rejection, or illness in group III. (a) Transplanted allogeneic total composite face/scalp flap at day 65 with nice hair without a sign of rejection. (b) Transplanted allogeneic total composite face/scalp flap (more than 100 days)
Complications
Two allograft recipients died at the early postoperative period in group III. No other isograft or allograft recipients died during the evaluation period.
Discussion
Soft-tissue deformities of the face and scalp may present as defects of a single component, such as skin, subcutaneous tissue, or muscle, or as defects of combinations of these parts. Extensive scalp loss due to burn or avulsion injuries is one of the most deforming and psychologically stressful conditions and creates a major reconstructive challenge. In case of severe tissue loss, it is virtually impossible to restore the normal appearance of the face and scalp. There is also deficiency of apt autogenous donor tissue sources with the desired texture and appearance required for optimal restoration of function and aesthetic. Thus, conventional facial restoration techniques might lead to skin discolorations, irregular textures, and perioral, perinasal, periauricular and periorbital contractures and cause an abnormal, expressionless and virtually mask-like face that is easily noticeable at conversation distance in most of the patients. These cases often require numerous reconstructive operations, nonetheless, the outcomes are not pleasing neither for the patient nor the surgeon.
After the first successful allogeneic hand transplantation in 1998 [21], various experimental and clinical studies of composite tissue transplants were performed for the reconstruction of the hand, knee joint, larynx, abdominal wall, tongue etc. [22–27]. These successful allotransplantation results opened the way for facial allotransplantation in human which was first performed in November 2005 in France by a team led by Professor Bernard Devauchelle and Professor Jean Michel Dubernard [28]. Since then, roughly 30 face transplantations have taken place in different parts of the world [29]. Though the surgical skills have been proven, the issue of immunotherapy remains as an obstacle essentially because this is not considered to be a lifesaving procedure [30, 31]. There are various defined experimental models for composite face/scalp reconstruction, including allogeneic transplantation [15–18, 23, 32–37]. Since there are some potential challenges during the allogeneic facial transplantation including the acute rejection, or failure of the anastomosis which would be a catastrophic situation, concomitant excision of the recipient’s own deformed face could be avoided. However the harvesting of the donor facial and/or scalp tissue is an emergent condition and cannot be delayed for a long time period. Thus would it be possible to harvest the donor facial tissues and keep them for a time period. It is not a probability to keep the harvested donor face in-vitro for a long time. Could it be possible to keep the harvested donor tissue at the body of the recipient in a heterotopic position? We know that the temporary ectopic replantation of the mutilated upper extremity before transferring to its original position was defined before and this technique is known as a babysitter procedure [20]. Is it possible to use babysitter procedure for the facial transplantation? To answer this question in an experimental fashion, we proposed to develop a heterotopic composite total face/scalp flap transplantation model in rats. We transplanted the harvested total face/scalp tissue of the donor rat to the back of the recipient rat. We preferred to transplant to the back of the rats to avoid auto-cannibalism. However, we think that anatomical factors will be more favorable among human subjects, for whom transplantation to the abdominal region could be possible without extensive morbidity and supplying a well hidden lower abdominal scar after the orthotopic transfer of the facial tissue. The scar would be as a tummy tuck scar. Moreover, during the partial facial transplantations the scar could be much shorter.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








