17 Free TRAM breast reconstruction
Synopsis
 The free TRAM is one tool in an entire armamentarium used for breast reconstruction.
The free TRAM is one tool in an entire armamentarium used for breast reconstruction.
 Although controversial, the free TRAM likely limits donor site morbidity and ischemic complications when compared with the pedicled TRAM.
Although controversial, the free TRAM likely limits donor site morbidity and ischemic complications when compared with the pedicled TRAM.
 Free TRAM reconstruction can be performed safely in an immediate or delayed fashion.
Free TRAM reconstruction can be performed safely in an immediate or delayed fashion.
 For the most part, radiation therapy after reconstruction yields more unpredictable results than radiation before reconstruction.
For the most part, radiation therapy after reconstruction yields more unpredictable results than radiation before reconstruction.
 Free TRAM flap breast reconstruction requires intraoperative attention to detail and postoperative vigilance.
Free TRAM flap breast reconstruction requires intraoperative attention to detail and postoperative vigilance.
 Although revision is not uncommon, the free TRAM provides for excellent, predictable aesthetic results with a high degree of patient satisfaction.
Although revision is not uncommon, the free TRAM provides for excellent, predictable aesthetic results with a high degree of patient satisfaction.
History
The origins of breast reconstruction roughly began a century ago in Europe, when Louis Ombredanne divided the head of the pectoralis minor muscle and turned it over to create a new breast mound.1 Interestingly, reconstruction in the United States was adopted more slowly due to Halsted’s belief that nothing should be done to the mastectomy site to hide or promote a recurrence. Although historical, the effects of Ombrédanne’s work were dwarfed by Tansini who first reported the use of the latissimus dorsi flap for breast reconstruction.2 His technique fell from favor, but resurfaced in the 1970s and is still used today. The use of serial tubed transfers of breast tissue from the healthy, contralateral breast, were popular during the 1940s and 1950s. The major drawbacks, which limited their longevity were their dependence on multiple operations to achieve a satisfactory final result and this came at the expense of a normal breast.
The use of alloplastic materials for breast reconstruction became popular in the 1970s and 1980s with the introduction of the silicone breast implant by Cronin and Gerow. This was soon followed by the use of a tissue expander by Radovan in 1982.3 The use of synthetic materials allowed for the reconstruction of the breast mound with excellent projection and symmetry without the concern for donor site morbidity. These devices were utilized for both immediate and delayed reconstruction.4,5 In many patients however, a mastectomy can result in the loss of an adequate soft tissue envelope to safely cover a prosthesis. As a result, the combination of a latissimus dorsi myocutaneous flap and implant became very popular for adequate volume without as much concern for healing under tension.6
Perhaps the most monumental contribution to the history of breast reconstruction came in 1982, when Hartrampf described the pedicled TRAM flap.7 This was the first use of a transverse island of skin and soft tissue from the abdomen for the reconstruction of a breast mound. Although this has largely taken second stage to free tissue transfer in recent times, the very nature of its dependence on redundant abdominal tissue provided for a new, yet lasting direction in the field of breast reconstruction.
The next jump in the history of breast reconstruction fell on the heels of the birth of microsurgery. Although Fujino and colleagues described the first use of free tissue transfer for breast reconstruction in 1976,8 the technique did not become popularized until the 1980s and 1990s. The shift toward increased dependence on free rather than pedicled transfer is based on the realization that the transverse skin island has a more robust blood supply from the deep inferior epigastric pedicle, which sends off perforating branches through the rectus muscles (this will be discussed in greater detail later in this chapter). This realization, in conjunction with improved microvascular technique and training, led to routine use of the free TRAM in many centers. Modifications of this technique include the muscle sparing free TRAM, the deep inferior epigastric perforator (DIEP) flap and the superficial inferior epigastric artery (SIEA) flap, but an understanding of the indications, technique and complications of the free TRAM provide the necessary foundation for understanding these more complicated procedures.
Basic science/disease process
One out of every eight women in the United States will develop breast cancer at some point in their lifetime. In total, roughly 2.5 million women in the United States are survivors of breast cancer, with this number expected to increase dramatically over the next decade.9 Given that mastectomy plays a major role in the treatment algorithm of breast cancer, it is not infrequent that women are faced with the decision of whether to have a breast mound reconstructed. In most cases, mastectomy offers the chance for cure and reconstruction provides a woman a better aesthetic result. In some cases however, even patients who have advanced metastatic cancer may be candidates for reconstruction, such as for radiation ulcers or nonhealing wounds.10 In addition, with the advent of genetic testing for BRCA I and II for risk stratification, an increasing number of women are electing to undergo prophylactic mastectomy.11 Likewise, the frequency of contralateral prophylactic mastectomy is increasing dramatically in patients who have undergone mastectomy for DCIS.12 As a whole, more and more reconstructive procedures are being performed annually in the United States.
The mastectomy defect can be devastating both physically and psychologically. Numerous studies have documented the significant improvement in self confidence and mental health following breast reconstruction.13–15 Autologous reconstruction in particular, has the benefit of replacing “like with like”, which in turn contributes to an improved feeling of restoration of the self after this devastating injury.16
Although breast cancer is by far the leading source of a thoracic defect requiring autologous reconstruction, it should not be forgotten that free TRAM reconstruction may also be used for defects such as chest wall sarcomas or lung cancer invading the chest wall where soft tissue coverage is needed after resection.17,18
Anatomy and physiology
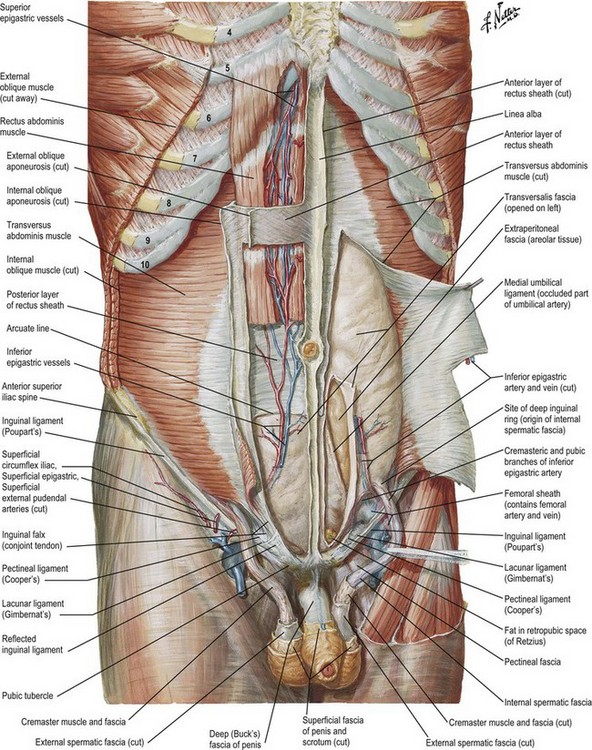
The anatomy of the abdominal wall is critical to a complete understanding of the differences between various forms of abdominal based breast reconstruction such as the pedicled TRAM, free TRAM, deep inferior epigastric perforator (DIEP), and superficial inferior epigastric artery (SIEA) flaps. The rectus abdominis muscles run in parallel on either side of the midline (Fig. 17.1). The origin is the symphysis pubis, whereas the insertion is the subcostal margin of ribs 5–7 and the xiphoid process. The recti are separated in the midline by the linea alba and crossed transversely by fibrous bands called tendinous inscriptions. The rectus muscles rest within the rectus sheath, a confluence of the aponeuroses of the external and internal oblique muscles, as well as the transversus abdominis. The arcuate line, a transverse line roughly one-third the distance between the umbilicus and the pubic symphysis, marks a transition in the composition of the rectus sheath. Above this line, the anterior rectus sheath is comprised of the external oblique and a portion of the internal oblique; whereas, the posterior sheath is comprised of a portion of the internal oblique and the transversus abdominis. Below the arcuate line, the posterior rectus sheath disappears and all three components of the aponeurosis pass anterior to the recti. Perhaps more importantly for the discussion of TRAM reconstruction, the arcuate line also marks the point where the inferior epigastric vessels perforate the rectus muscles.
The blood supply of the lower anterior abdominal wall comes from both the superior and inferior deep epigastric arteries which connect to one another via “choke” vessels in the mid-abdomen (Fig. 17.1). The arteries send perforating vessels through the rectus muscle to the soft tissue and skin of the abdominal wall. Having two dominant pedicles, this rectus is a Mathes and Nahai type III muscle.19 The typical TRAM flap utilizes skin and soft tissue from infra-umbilical redundancy; therefore, when harvesting a pedicled TRAM flap, the survival of the flap island is dependent on the choke vessels at the end of the superior epigastric artery. As a result, the flap runs the risk of ischemia. A free TRAM has the benefit of a more robust blood supply because the perforators off of the inferior epigastric artery offer a more direct route of blood flow. In addition, because these perforators can often times be visualized, some of the rectus muscle may be salvaged.
Diagnosis/patient presentation
Occasionally, patients may present to the plastic surgeon de novo; however, more commonly patients present as referrals from their surgical oncologist or general surgeon. Unfortunately, many cancer patients are never referred to a plastic surgeon to discuss reconstruction options. In one survey, only 24% of general surgeons referred greater than 75% of their patients for reconstruction.20 In addition, once referred, the best outcomes are ensured by an open dialogue between the oncologic and plastic surgeon with regards to incision planning and skin flap creation. As a result, the overall approach to breast cancer and subsequent reconstruction to ensure excellent results and patient satisfaction should be multidisciplinary. That being said, patients present to the office in various stages of their cancer treatment and one must be aware of the implications, most notably timing of reconstruction with respect to mastectomy and radiation therapy.
The pedicled versus free TRAM
The main issues at stake when comparing these two techniques are: the technical aspects of the operation, the long term results, and the donor site morbidity. The pedicled TRAM requires complete dissection of the rectus muscle up to the level of the xiphoid (see Ch. 15). The pedicle and flap are then transposed through a tunnel in the most medial aspect of the inframammary fold. Because the flap and pedicle are turned over, there is the risk of twisting; thus, the insetting of the flap itself can be quite challenging. It is also not unusual to have contour irregularities along the inferomedial mammary fold and symmetry may be more difficult to achieve.21,22 The free TRAM on the other hand requires the additional expertise of a microanastomosis; however, once the pedicle is created, the insetting of the flap tends to be less problematic.
Aside from the technical aspects of the two operations, one must compare the long term results weighed against the donor site morbidity. Due to the reliance on “choke” vessels for flap survival in the pedicle TRAM, there is a theoretical increased risk of ischemic complications such as partial or total flap loss and fat necrosis. This theory has been put to the test in multiple studies, but results are conflicting. Andrades et al. compared 147 pedicled TRAMs to 154 free TRAMs of various muscle sparing (MS) iterations. MS0 connotes complete transection of the muscle; whereas, MS1 involves transection of most of the muscle, MS2 only the central portion and MS3 (DIEP) none. This showed a significant increase in mild and severe fat necrosis in pedicled TRAMs compared to MS0 free TRAMs. Interestingly, evaluation of MS1 and MS2 flaps suggest that as the degree of muscle sparing increases, so does the rate of fat necrosis. In fact, there does not seem to be a significant difference in flap necrosis between pedicled TRAM and MS2 free TRAM. There was no significant difference in partial or total flap loss.23 Alderman et al. prospectively followed patients who underwent expander/implant, pedicle TRAM and free TRAM over a 2-year period. They showed no significant differences in complication rates between pedicled and free TRAM.24
Although sacrificing the rectus muscle will not leave a patient completely disabled, patients may notice a considerable difference in flexion strength and abdominal contour when the rectus muscles are sacrificed. Research on abdominal wall strength comparing pedicled to free TRAM has been conflicting. Objective measures of abdominal wall strength after pedicled or free TRAM reconstruction have consistently shown a deficit in strength which may persist long term. Following pedicled TRAM, one study has shown that patients may experience on average up to a 23% deficit in trunk flexion, but this may be as high as 40% in bilateral reconstruction. In unilateral free TRAM, this deficit may be up to 18%.25 However, multiple studies have tried to evaluate pedicled versus free TRAM head to head and none have been able to show a significant difference in long term abdominal wall function.26,27 This is likely due to the fact that during a standard free TRAM, the entire width of the lower rectus muscle is sacrificed. Further studies are needed to differentiate long term abdominal wall function comparing MS0, MS1, MS2 and DIEP flaps.
Radiation therapy
Radiation therapy is a component of multimodality therapy for many types of cancers. Current guidelines support using radiation therapy for breast cancer in the adjuvant setting following lumpectomy for DCIS, with the possible exception of small tumors with a focus of low grade tumor that has been resected with wide margins. In patients with early stage (T1–2) invasive cancer undergoing breast conservation, radiation therapy decreases the risk of local recurrence in patients who are node negative or positive; therefore, it is offered to patients who are node negative with the exception of those individuals over the age of 70 and are hormone receptor positive. Patients with four or more positive lymph nodes benefit from irradiation to the remaining nodal basin as well.28 Aside from its use in breast conservation therapy, radiation therapy is also used as adjuvant therapy following mastectomy. In this setting, patients who have close surgical margins, four or more positive lymph nodes or are clinical stage III or IV benefit from radiation therapy.29 Traditionally, radiation therapy has been used to decrease local recurrence. Although this remains its primary benefit, recent studies have suggested a modest albeit, improved survival benefit with radiation in both breast conservation and mastectomy patients.30 In addition, studies have suggested that one to three positive lymph nodes may be an indication for radiation therapy in certain patients and as such, radiation is increasingly being utilized. More studies are needed for a consensus on management of these patients.31 Taken as whole, more and more patients are currently being offered radiation therapy making knowledge of its effects paramount to the reconstructive plastic surgeon.
In the reconstructed breast, radiation may play havoc, especially following tissue expansion/implant reconstruction which is prone to capsular contracture and asymmetry. Aside from poor aesthetic outcomes, contracture can be so severe, patients are at risk for chronic pain and implant extrusion. Autologous reconstruction, although significantly better than prosthetic reconstruction when faced with irradiation, still is at higher risk of complications when compared to non-irradiated patients.32,33
Nevertheless, patients will continue to need irradiation and until a less invasive but effective treatment alternative surfaces, the main question will remain: does the timing of radiation therapy in relation to reconstruction matter? The answer to this question deals with two topics. First, and perhaps most importantly, reconstruction should at no point compromise the treatment of the breast cancer, nor sacrifice the efficacy of the radiation therapy itself. Retrospective studies have found acceptable outcomes following immediate reconstruction followed by irradiation; however, no large prospective study has ever attempted to answer this question.34 Although the local recurrence rate has never been shown to be higher, the ideal radiation field may be compromised following immediate reconstruction.35 Motwani et al. were able to show that autologous breast reconstruction did compromise radiation delivery in 52% of immediate reconstruction patients, this in comparison to 7% of age matched controls.36 Although the radiation field design required changes, the clinical significance of this remains unknown.
An alternative to immediate reconstruction followed by radiation is the so-called “delayed-immediate” breast reconstruction.37,38 The postoperative plan for adjuvant therapy is often unknown prior to mastectomy as it is ultimately based upon the tumor characteristics and node status of the final pathology. However, patients who definitely will need post mastectomy radiation therapy are ideal candidates for this option. In this method, patients undergo a mastectomy immediately followed by subpectoral tissue expander placement. The tissue expander is partially inflated in the operating room and further postoperatively in an effort to maintain the volume of the native breast skin envelope. The expander is deflated in order to undergo radiation without a compromised field and subsequently reinflated after radiation in complete. Several weeks after radiation, when the effects of the radiation have matured, the patient returns to the operating room for removal of the tissue expander and definitive reconstruction with autologous tissue. Although this method is increasingly being used at several medical centers, the overall benefits of this option have yet to be fully elucidated.
Patient selection
Risk factors
From the SEER database, the peak incidence of breast cancer in the United States is age 61, with almost 60% of all cases of breast cancer under age 65.39 The significance of this is that the majority of women tend to be fairly young and without major co-morbidities. As a result, it is exceedingly rare for a woman to be considered too high risk for surgery; rather, understanding the predictors of poor outcomes consequently has more to do with proper patient education for expectations and to modify risk wherever possible.
In a review of 500 free flaps, Selber et al. were able to demonstrate an increased risk of wound infection, mastectomy flap necrosis, abdominal flap necrosis and fat necrosis in smokers. In addition, obese patients are more likely to experience wound related complications including mastectomy flap necrosis. Peripheral vascular disease was identified as a risk factor for wound infection.40 Greco et al., in a similar review, did not corroborate the smoking data; however, they too found obesity to play a major role in wound related complications.41 Although not definitively shown in the breast free flap literature, smoking cessation preoperatively has been shown to decrease wound related complications following surgical procedures in general. Although, adequate soft tissue is necessary to be able to use the transverse island of tissue for reconstruction, the obesity end of the spectrum demonstrates that too much of a good thing clearly leads to poor outcomes. Patients undergoing breast reconstruction are usually either confined by the timing of their mastectomy or eager for reconstruction making the prospect of preoperative weight loss to improve outcomes unlikely. Although diabetes mellitus has long been looked at as a harbinger of surgical complications, in free flap reconstruction, it has not been shown to increase risk of poor outcomes. Miller et al., in their review of 893 free flaps, failed to show a significant difference in outcomes when comparing type I, type II and nondiabetic patients.42 That being said, there is a substantial bit of literature suggesting that tight glucose control in the postoperative period decreases wound related complications.43 Although it is unclear if this is generalizable to free flap reconstruction, given the body of literature, attention should be paid to this detail in the postoperative period.
Prior abdominal operations have been shown to increase the risk of complications associated with TRAM flap reconstruction.44 Most importantly, prior transversely oriented incisions might be an indicator that the epigastric vessels have been sacrificed. Techniques for minimizing risk include skewing the abdominal flap away from the previous scar, using hemiflaps, minimizing flap undermining, and supercharging.45 Similarly, a prior abdominoplasty is generally considered an absolute contraindication to TRAM flap reconstruction because the prior skin flap sacrifices all perforating vessels. Although there are reports of successful TRAM following abdominoplasty, this should not be attempted without considerable expertise and a complete analysis of other options.46 A thorough knowledge of abdominal wall vascular anatomy, the likely effects of prior standard abdominal incisions as well as the proposed surgery all contribute to an overall successful endeavor.