8 Foot reconstruction
Synopsis
 Successful wound healing requires determination of wound etiology, ensuring adequate blood supply and proper identification and treatment of infection.
Successful wound healing requires determination of wound etiology, ensuring adequate blood supply and proper identification and treatment of infection.
 This requires a team effort involving at the very least a vascular surgeon, a foot and ankle surgeon, a plastic surgeon, a diabetologist, an infectious disease specialist, a pedorthotist and a prosthetist.
This requires a team effort involving at the very least a vascular surgeon, a foot and ankle surgeon, a plastic surgeon, a diabetologist, an infectious disease specialist, a pedorthotist and a prosthetist.
 Surgical incisions and flap design relies upon a detailed knowledge of anatomy, angiosomes, and vascular status.
Surgical incisions and flap design relies upon a detailed knowledge of anatomy, angiosomes, and vascular status.
 Adequate debridement of the wound is critical before attempting a reconstruction. This may require a staged procedure.
Adequate debridement of the wound is critical before attempting a reconstruction. This may require a staged procedure.
 The best solution for a successful wound closure must be tailored to the patient’s age and functional capacity.
The best solution for a successful wound closure must be tailored to the patient’s age and functional capacity.
 The key is to pick the optimal biomechanical result given the patient’s age and functional capacity. In one patient, that may involve a free flap reconstruction while in another, it may involve a below the knee amputation,
The key is to pick the optimal biomechanical result given the patient’s age and functional capacity. In one patient, that may involve a free flap reconstruction while in another, it may involve a below the knee amputation,
 A successful reconstruction may ultimately fail without appropriate postoperative offloading techniques to allow healing and without addressing the biomechanical abnormalities that led to the initial breakdown.
A successful reconstruction may ultimately fail without appropriate postoperative offloading techniques to allow healing and without addressing the biomechanical abnormalities that led to the initial breakdown.
Historical perspective1
The history of modern lower extremity wound therapy is an unfortunate chronicle of widespread ablation and aggressive amputations. Dating back to 1400 bc, Ancient Egyptian papyrus records note some of the first attempts at clinical observation to manage disease and wounds by application of liquid remedies, honey, grease, and lint.2–4 Greek manuscripts such as the records of Hippocrates (460–377 bc), detail wound irrigation, the use of topical vinegar, and the application of protective dressings; seminal principles that have endured for millennia. The syringe was invented by a Greek barber in 280 bc and was noted to be good for injecting liquids and sucking pus out of wounds. The Greek’s name for the syringe is pyúlkos, latinized as pyulcus, the “pus-puller”, a useful instrument forgotten for almost 2000 years.5 Galen, a Roman surgeon coined the mistaken term “pus bonum et laudabile” (good and commendable pus), as he observed that pus from the wounds of stricken gladiators preceded healing. This incorrect association between pus and healing would persist for centuries to the extent that foreign material, associated with increased pus production was encouraged. Ambroise Paré was the official royal surgeon for kings Henry II, Francis II, Charles IX, and Henry III, and is credited with the first accounts of upper and lower extremity prostheses, maggot therapy, and vascular ligation during amputation. One of Napoleon’s surgeons, Dominique Jean Larrey (1766–1842), advocated early amputation for any limb injured that could not be saved reasoning that early amputation might create a clean viable wound.6 During the American Civil war, soldiers wounded on the battlefield received whiskey for shock; early amputation of devitalized tissue, and ligatures such as boiled horse hair that was used to tie off major vasculature of the extremities. Sutures were left in place until the formation of “laudable pus” around day seven to nine, at which point the suture was pulled from the wound. With major amputation rates approaching 90%, outcomes were predictably disappointing.7
Expanding on the seminal ideas of Koch (1843–1910) on microbial growth, Joseph Lister (1827–1912) and Louis Pasteur (1822–1895) first advocated antisepsis and the use of carbolic acid (phenol) on open wounds to prevent infection and decrease amputation rates. A Russian military surgeon named Carl Reyher (1846–1890) further recommended adding a vigorous mechanical wound cleansing, which he termed debridement, which resulted in a significant reduction in patient mortality during the Russo-Turkish war.8 French surgeon Pierre Joseph Desault (1744–1795) proposed that inflammation from injury produced constriction of soft tissues confined by fascia or aponeuroses, what he termed “étranglement”, a precursor to gangrene. Debridement (“to unbridle”) was used by making an incision through investing fascia to release the underlying expanding tissue. Combat continued to advance the treatment of wounds. During the First World War, a Belgian surgeon, Antoine Depage (1862–1925) promoted sharp wound debridement, delayed wound closure, and relied on microbiological assessment of wound brushings as a guide for the timing of secondary wound closure, all sound principles that laid the foundation for modern wound therapy.
Basic science
Angiosomes of the foot
In the normal patient, blood flow to the foot and ankle is redundant with three major arteries feeding the foot via multiple arterial–arterial connections. By having a thorough grasp of those arterial–arterial interconnections and by selectively Dopplering out those connections, it is possible to map out the existent vascular tree including the direction of flow. By adding the concept of angiosomes, the vascular supply becomes even clearer. G. Ian Taylor first originated the concept of angiosomes in human anatomy to describe a three-dimensional unit of tissue supplied by a single source artery. Attinger et al.1 further developed this principle as it applies to the foot and ankle, eventually illustrating six specific foot angiosomes originating from three primary source arteries. All vascular supply to the foot and ankle enters through the popliteal fossa and the terminal branches of the popliteal artery: the anterior tibial, posterior tibial and peroneal arteries.
Above the ankle, the peroneal artery bifurcates into the anterior perforating branch and calcaneal branch. The anterior perforating branch of the peroneal artery, goes through the anterior distal intermuscular septum between the peroneal and tibial bone and sends a branch superiorly overlying the intermuscular septum (this area encompasses the area from which the supra-malleolar flap9 can be harvested). The anterior perforating artery then connects directly with the anterior lateral malleolar artery and supplies the anterolateral ankle angiosome. The lateral calcaneal artery begins at the level of the lateral malleolus as it emerges laterally between the Achilles tendon and the peroneal tendons. It curves with peroneal tendons 2 cm distal to the lateral malleolus and gives rise to four or five small calcaneal branches. The lateral calcaneal artery terminates at the level of the fifth metatarsal tuberosity, where it connects with the lateral tarsal artery. The calcaneal branch supplies the lateral and plantar heel angiosome.
The six angiosomes can be used to optimize the success of any planned treatment or procedure. The incision can be placed along the boundary of two adjoining angiosomes to ensure that the blood flow on either side of the incision is optimal. Flaps can be dissected out with a high degree of reliability. Amputations can be performed in a way that minimizes the risk of distal tissue necrosis. The revascularization can be planned to increase the chance of healing of a given wound or ulcer by 50%.10 This detailed knowledge of vascular anatomy helps take the guesswork out of limb salvage. The surgeon is now able to determine which leg is salvageable and which has a significant chance of failure. He/she can also choose which mode of reconstruction has the most chance of success, given the existing blood supply. He/she can determine when salvage is not possible and go right to an amputation. This spares the patient needless, costly and potentially dangerous procedures, with little chance of success.
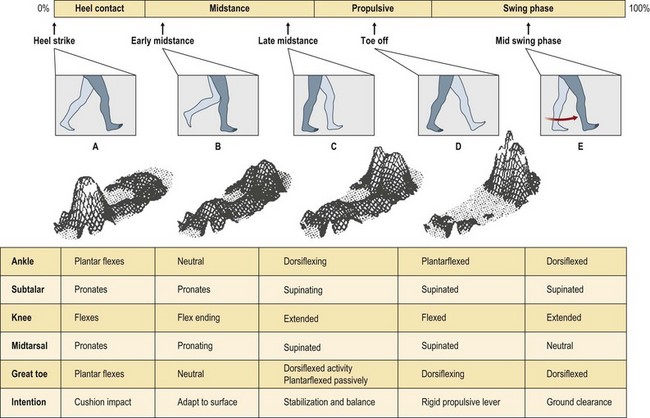
Gait analysis
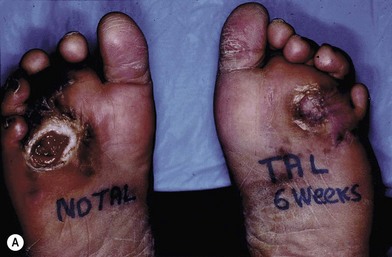
When looked for, abnormalities of gait can be detected in most patients evaluated for soft tissue repair. Working with a podiatrist and a foot an ankle surgeon is critical to determine what skeletal or muscle abnormality exists so that it can also be addressed when treating the wound. Plantar forefoot ulceration in diabetics is usually due to shortening of the Achilles tendon with resultant increases in weight-bearing pressures over the plantar foot during ambulation. Other possible causes that need to be ruled out or treated include hammer toes, long metatarsal, hallux rigidus, etc. The release of the tight Achilles tendon results in rapid healing, although at the cost of a permanent decrease in push-off forces. This however, offers long-term protection, decreasing ulcer recurrence rates by 75% at 7 months and 54% at 25 months from surgery (Fig. 8.1).11,12
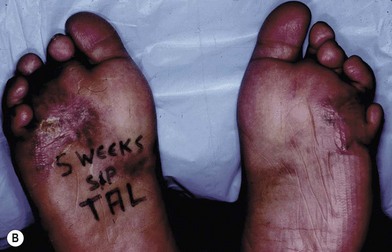
Preoperative evaluation of gait should include a measure of ankle dorsiflexion as well as an F-scan analysis. When evaluating for equinus deformity, the leg must be held first in complete extension in an effort to keep the gastrocnemius muscles at their full resting length and then with the knee flexed to assess the soleus muscle. If the ankle only dorsiflexes with knee flexion, then the gastrocnemius muscles are responsible for the tightness and can be released by performing a gastrocnemius recession. If the ankle remains tight both in extension and flexion, then both portions of the Achilles tendon have to be released, usually percutaneously. F-scan gait analysis uses multiple pressure sensing surface probes that record pressures on the sole of the foot during all phases of gait. Areas of excessive compression during weight-bearing can be displayed in graphical format and help plan the biomechanical components of the required reconstruction, as well as subsequent orthotics (Fig. 8.2). The changes which occur with tendon transfer or lengthening procedures may be documented, as well, with this technique.
Patient presentation
Connective tissue disorders
The connective tissue disorders (e.g., systemic lupus, rheumatoid arthritis, and scleroderma) are frequently associated with Raynaud’s disease that causes distal vasospasm and cutaneous ischemia leading to lower extremity ulcers. The treatment of these connective tissue disorders frequently requires immunosuppressive drugs such as steroids or chemotherapy agents that can further inhibit healing. Although the wound retarding effects of steroids can be partially reversed with oral vitamin A (20 l000 U/day every other day while the wound is open), systemic use of vitamin A may sufficiently counter the effects of therapeutic effects steroids and this may not be desirable. Topical vitamin A at 1000U/g of ointment is usually sufficient to improve wound healing. In addition, almost half of vasculitis patients suffer from a coagulopathy leading to a hypercoagulable state. This can include any combination of Factor V Leiden deficiency, Protein C and/or S deficiency, antithrombin III deficiency, PAI 1 mutation, antiphospholipid syndrome, etc. Therefore, a coagulation blood panel should be obtained on these patients and if abnormalities exist, they should be treated with anticoagulants by the hematologist. Injectable heparin moieties, such as Lovenox, have proved to be far more effective than warfarin in healing these ulcers.13 The treatment of these ulcers is principally medical. Once the abnormalities have been identified and corrected, wound healing adjuncts can help in healing the wound.
Ischemia
Endovascular surgery has revolutionized lower extremity revascularization. This technique allows the surgeon to dilate arterial narrowing, open up an artery and keep the treated arterial segment open using drug eluting stents, or create a new subendothelial channel to reroute the blood. They are able to re-canalize occluded arteries using laser, routing, cryotherapy techniques or creating a new channel by piercing the intima. Endovascular surgery is all the more attractive because bypass surgery carries a 15–20% complication rate at the incision sites. The results of endovascular surgery are rapidly approaching those of the “gold standard”, bypass surgery.14 In bypass surgery, venous conduits remain the standard, but the availability of drug-coated grafts and the venous patch technique with or without AV fistula15,16 are now providing an effective alternative. Combining endovascular and bypass techniques is very effective because shorter bypasses are required. It is important to remember that optimal tissue oxygenation around a wound after bypass surgery takes 4–10 days, while with endovascular surgery it can take up to 28 days. Premature wound closure attempts fail to take full advantage of the revascularization.
Brachial artery systolic pressure generally serves as a reference for determining the ankle/brachial index and gives an indication of the relative degree of ischemia. In addition, the absolute pressure measured at the ankle correlates well with the healing potential of most soft tissue wounds in the foot. A pressure cuff of 12 cm in width may be placed at the ankle level. By listening with the hand-held 10-MHz Doppler probe over the dorsalis pedis and posterior tibial vessels, the pressure at which the flow is occluded may be recorded and compared with the brachial artery occlusion pressure. In general, a normal ankle brachial index (ABI) is 1.0 or slightly greater. ABIs exceeding 0.7 have been felt to be “acceptable” for most reconstructive techniques. In diabetics, an ABI below 0.9 mandates further evaluation. Patients with indices of 0.3 or lower have rest pain, nonhealing wounds, or both. One review of vascular disease patients suggested that an ankle/brachial index of <0.5 indicates the need for a revascularization procedure before undertaking a complex reconstruction. The decision, however, is multifactorial and generally requires a complete extremity evaluation.17 Absolute pressure measurements of <50 mmHg signify severe arterial disease with a poor prognosis for healing,18 although neither the absolute pressure, nor the ankle/brachial index are always accurate.19 Noncompressible arterial walls are seen in 5–30% of patients with diabetes mellitus and are caused by medial calcification, which falsely elevates the values for both tests.
Lassen et al. demonstrated that absolute toe pressures provide a highly accurate method for determining the likelihood of healing in the affected foot.18 Barnes et al. and others20,21 would agree that toe pressures in diabetic patients predict healing potential. A toe pressure with pressure <30 mmHg represents severe ischemia that requires vascular bypass surgery.22
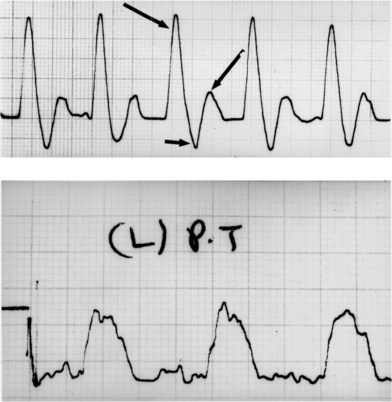
Directional Doppler flow studies are extremely important in assessing the status of the peripheral circulation. Although many approaches to quantitative analysis of waveforms have been suggested, adequate assessment of waveforms is provided by qualitative evaluation.23 The normal triphasic waveform becomes abnormal distal to an obstructing vascular lesion. As the obstruction becomes more significant, the waveform may deteriorate from triphasic to biphasic with a reversed flow component, to biphasic, to monophasic, to aphasic (Fig. 8.3). All patients with aphasic waveforms and foot wounds should undergo vascular surgery before attempts at reconstruction. Patients with triphasic or either of the two forms of biphasic flow generally do not require vascular reconstructive procedures. Limbs with monophasic flow require further study, depending on the location of the wound and the complexity of the proposed reconstruction. If microvascular composite tissue transplantation is required for wound closure, one should attempt to obtain at least biphasic or “good” monophasic flow in the recipient vessel before the reconstruction. A narrow complex with a sharp upstroke and good amplitude characterizes “good” monophasic signals. If there is any question as to the quality of blood flow, an angiogram should be obtained. If local flap or skin-grafting techniques are employed, additional information regarding skin blood flow is needed before vascular surgical intervention can be deemed necessary.
Diabetic foot ulcer
A total of 24 million (7.8%) of all Americans have documented diabetes mellitus and 15% of them eventually develop a foot ulcer during their lifetime.24 Almost 15% of the healthcare budget of the US goes toward management of diabetes, with 20% of hospitalizations and 25% of diabetic hospital days for the treatment of diabetic foot ulcers.25 Two-thirds all the major amputations performed per year in the US are performed in diabetics.26 Diabetics battle numerous complications related to their underlying disease, but none is more devastating, both psychologically and economically, than gangrene of an extremity and its associated risk of amputation.
Diabetic peripheral polyneuropathy is the major cause of diabetic foot wounds. More than 80% of diabetic foot ulcers have some form of neuropathy present. The neuropathy is a consequence of chronically elevated blood sugars that cause vascular and metabolic abnormalities. Elevated intraneural concentrations of sorbitol, a glucose byproduct, are thought to be one of the principle mechanisms for nerve damage. Further damage can result when the damaged nerve swells within anatomically tight spaces such as the tarsal tunnel. The combination of nerve swelling and tight anatomic compartments leads to the “double crush syndrome,” which can sometimes be partially reversed with nerve release surgery.27 Unregulated glucose levels elevate advanced glycosylated end-product (AGE) levels that may induce microvascular injury by cross-linking collagen molecules. Decreased insulin levels, along with altered levels of other neurotrophic peptides, may decrease maintenance or repair of nerve fibers. Other potential causative factors of peripheral polyneuropathy include altered fat metabolism, oxidative stress, and abnormal levels of vasoactive substances such as nitric oxide.
Hyperglycemia can also affect the body’s ability to fight infection. Hyperglycemia diminishes the ability of polymorphonuclear leukocytes (PMNs), macrophages and lymphocytes to destroy bacteria. In addition, the diabetic’s ability to coat bacteria with antibiotics is diminished, which further helps shield bacteria from phagocytosis. As a result of this impaired immune state, diabetics are especially prone to Streptococcus and Staphylococcus skin infections. Deeper infections tend to be polymicrobial, with Gram-positive cocci, Gram-negative rods, and anaerobes being frequently present on culture. PCR has shown that there are at least 39 species within the biofilm of such wounds, with over 60% being anaerobic. Postoperative complication rates correlate directly with the level of postoperative hyperglycemia.28,29 Finally, diabetics have decreased stem cells in their bone marrow and decreased stem cell pluripotency when mobilized.30 This affects the healing of wounds.
Some 80–85% of amputations are preceded by nonhealing ulcers in patients with neuropathy.31,32 Despite attempts to decrease the number of amputations in the US by various strategies from better glucose control, to monitoring screening exams for impaired sensibility, the number of amputations has continued to increase from 54 000 in 1990 to 71 000 in 2004.33,34 In spite of a better understanding of the pathogenesis of foot ulceration in the diabetic and the institution of a multidisciplinary approach to management, trends in amputation have not shown any tendency towards improvement in recent years and foot ulceration remains the most common reason for hospitalization of the diabetic patient in the US.35–38
Foot ulceration is much more common in those patients with neuropathy and vascular disease: the annual incidence rises from <1% in patients who do not have neuropathy, for example, to more than 7% in those with established neuropathy.39,40 The average cost of an ulceration was $27 500 in 1997 and the cost of an amputation ranged from $22 702 for a toe, to $51 281 for a leg, with the annual cost for diabetic neuropathy and its complications in the US being between $4.6 and $13.7 billion.41,42 Once an amputation has been performed, the incidence of a second amputation in the contralateral limb approaches 50% within 2 years.43–45
Several misconceptions have perpetuated a “fatalistic” approach towards the management of the diabetic foot resulting in excessive amputations over the years. The first is that all diabetic foot problems are due to “small vessel disease.” Specifically, the presumption is that distal arteriolar occlusive disease can cause ischemic wounds even in the presence of normal pedal pulses. This misconception dates back to the work of Goldenberg more than 40 years ago.46 More recent studies do not corroborate this retrospective analysis, including a blinded study by Strandness performed in a prospective fashion.47 Other prospective evaluations using arterial casting techniques also failed to show diabetic-specific distal arterial occlusions.48 Blood flow studies in diabetic patients undergoing femoral-popliteal bypass have shown no difference in the responsiveness of the runoff bed to papaverine vasodilatation, when compared with the same measurements made in nondiabetic patients, indicating normal reactivity of the resistance vessels (the arterioles).49 The second misconception is that endothelial cell proliferation occurs within the small vessels of diabetic patients and thereby, results in small vessel occlusions. Prospective studies have failed to show an increase in the incidence of intimal hyperplasia in the small vessels of these individuals.50 Thickening of the capillary basement membrane has been well documented, but capillary narrowing or occlusion has not.
Although the infrapopliteal arterial occlusive disease present in diabetic patients often results in distal vascular insufficiency, it is now well accepted that peripheral neuropathy is the primary cause of foot wounds in the diabetic population.51 The lack of a PO2 gradient between arterial blood and foot skin among diabetic patients without ulceration, diabetic patients with ulceration, and nondiabetic patients with normal transcutaneous oxygen tension further implicates a nonischemic etiology.52
Neuropathic changes
Charcot deformities (neuroarthropathy) of the small joints of the foot occur in 0.1–2.5% of the diabetic population.53,54 When present, the tarsometatarsal joints are involved in 30%; the metatarsophalangeal joints in 30%; the intertarsal joints in 24%; and the interphalangeal joints in 4% of the time. The explanation for these degenerative changes is widely debated. One theorized etiology is “neurotraumatic,” i.e., joint collapse occurring as a result of damage that has accumulated because of insensitivity to pain, although small fiber functions may be preserved.55 The destructive changes that occur in the Charcot foot cause a collapse of the medial longitudinal arch, which alters the biomechanics of gait. The normal calcaneal pitch is distorted which in turn causes severe strain to the ligaments that bind the metatarsal, cuneiform, navicular, and other small bones that form the long arch of the foot.56 These degenerative changes further alter the gait, resulting in abnormal weight bearing stress, causing a “collapse” of the foot. Unfortunately, ulceration, infection, gangrene, and limb loss are frequent outcomes if the process is not halted in its early stages.
Histologic similarities between chronically entrapped nerves and those seen in diabetic neuropathy provide substantial evidence that diabetic patients are unusually susceptible to peripheral nerve compression.57,58 Several authors suggest that nerve compression (subclinical) in concert with early diabetic nerve changes (subclinical) may give rise to a clinical picture of diabetic neuropathy (the “double crush hypothesis”) and therefore advocate peripheral nerve release at known anatomic sites of compression.59
Hemorheologic abnormalities
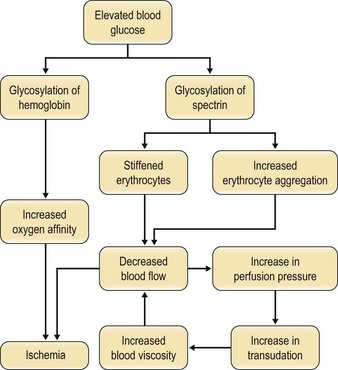
Although “small vessel disease” has not been anatomically confirmed in diabetic patients, recent evidence suggests that a functional alteration in capillary blood flow probably exists. This abnormal hemorheology may be responsible for much of the clinical picture now defined as “diabetic complications,” i.e., retinopathy, nephropathy, microangiopathy, and neuropathy.60 Increases in blood and serum viscosity and flow abnormalities in leukocytes, erythrocytes, platelets, and plasma proteins have been reported in diabetic patients.61 Many investigators equate these hemorheologic impairments as the functional equivalent of diabetic microangiopathy.62,63
The increase in blood viscosity noted in diabetic patients appears to have its origin in a stiffened red blood cell membrane as well as an increase in erythrocyte aggregation.64–66 Since red blood cells must deform to pass through capillary beds, stiffened cells may resist passage and even traumatize the endothelium. The nonenzymatic glycosylation of the red blood cell membrane protein spectrin is responsible for the membrane stiffening and the increased aggregation that occurs.67 Both of these result in an increase in blood viscosity. The mechanism of this glycosylation is similar to that seen with hemoglobin and is directly proportional to serum glucose levels.68,69
The altered flow characteristics that result from changes in viscosity trigger a compensatory rise in perfusion pressure, causing an increase in transudation across capillary beds and further increases in viscosity. Ischemia to the peripheral tissues is further exacerbated by the increased affinity of glycosylated hemoglobin for the oxygen molecule. The detrimental effects of hyperglycemia on blood flow and tissue perfusion are significant (Fig. 8.4).
Fortunately, many of these effects may be reversed by establishing good metabolic control of the diabetes. Juhan et al. found that the stiff red blood cell in the uncontrolled diabetic could be reversed to normal within a 24-h controlled insulin infusion.70 Pentoxifylline, a trisubstituted methylxanthine derivative, improves the deformability of erythrocytes and may be of considerable benefit to diabetic patients with extremity wounds. Pentoxifylline increases adenosine triphosphate (ATP) levels within red blood cells. Because erythrocyte deformability is ATP-dependent,71 the net result is a more flexible erythrocyte. Both in vitro and in vivo studies have documented measurable improvements in blood viscosity, as well as extremity blood flow, in diabetic patients with peripheral vascular disease treated with pentoxifylline.72–74 The salutary effects of long-term administration of pentoxifylline to type I and II diabetic patients with microvascular (retinopathy and nephropathy) and macrovascular (ischemic heart disease and peripheral vascular disease) complications have been well documented.75 A few studies have demonstrated the efficacy of cilostazol as an adjunct treatment of ulcers; however, the trials are small and without control groups.76,77 Cilostazol is a selective inhibitor of 3-type phosphodiesterase (PDE3) increases cAMP and the active form of PKA, which is directly related with an inhibition in platelet aggregation.
Chronic wound evaluation
The wound is assessed carefully by measuring its size and depth and then photographed. A metallic probe is used to assist in the evaluation of the depth of the wound. If the probe touches bone, there is an 85% chance that osteomyelitis78 is present. If tendon is involved, the infection is very likely to have tracked proximally or distally. Note the presence and extent of cellulitis and differentiate this from dependent rubor. The blood flow to the area is then evaluated by palpation and/or hand held Doppler. If the flow is inadequate, the patient should then be referred to a vascular surgeon. It is important to consider converting a “chronic wound” into an “acute wound,” via surgical debridement that includes removing not only the wound base covered with excessive matrix metalloproteinases and biofilm but also the wound edges that usually consist of senescent cells. By removing the factors inhibiting the chronic wound from healing and by correcting the local and systemic factor that may also have contributed, the wound then has a chance of healing.
Bone assessment
The precise evaluation of the extent of sequestra in patients with chronic osteomyelitis can be a formidable problem. Isosulfan blue dye may be used to help determine viable from nonviable bone. This dye has been used either via systemic administration79 or regionally through a superficial vein.80 The regional technique allows one to use a lower total dose of the dye and a lower concentration. An intravenous catheter is placed through a superficial vein on the dorsum of the foot. An ankle tourniquet is then inflated and 10 mL of 0.1 % isosulfidine (isosulfan) blue dye is injected followed by catheter removal. Over a period of minutes the bone and soft tissues of the foot become stained with the dye. The contrast between unstained, avascular bone and soft tissue and the stained tissue is readily apparent, thus facilitating precise debridement techniques
Infection identification and directed antibiotic therapy
Wound infection is characterized by the presence of replicating microorganisms within a wound, resulting in a subsequent host response such as erythema, warmth, swelling, pain, odor, and purulent drainage. If not recognized and treated, the infection will spread systemically leading to fever, an elevated white blood cell count, and possibly sepsis. The threshold between colonization and infection may be minute, so early identification and action are critical to healing wounds and minimizing complications. Quantitative research has shown that when the bacterial count (bioburden) reach levels more than 105 bacteria/g of tissue, a wound infection will result and inhibit the wound healing process.81
If the wound is acutely infected with draining purulence, odor emanating from it and/or with proximally ascending erythema, it needs to be debrided immediately and very aggressively to prevent sepsis and possible limb loss or death. The involved compartments of the extremity should be released if there is any question that the compartment pressures are abnormally high. Wounds with foul-smelling odors should be debrided sufficiently to get rid of the odor. If the foul odor is still present at the end of the procedure, then the debridement is incomplete and more necrotic or infected tissue should be excised. It is important to remember that just because an extremity presents with gas gangrene, it should not be summarily amputated, as aggressive, repetitive debridement ± hyperbaric oxygen, can often salvage enough of the limb to preserve a functional extremity. Hyperbaric oxygen has been shown to be particularly helpful in the control of anaerobic infections.82
Aerobic and anaerobic cultures of the wound are obtained during the debridement by taking pieces deep to the surface of infected tissue and purulence. A swab or superficial tissue culture is of limited use, because it usually reflects surface flora rather than the actual underlying bacteria responsible for the infection. One should then debride the wound as specified above and start broad-spectrum antibiotics after the deep tissue cultures have been obtained. The antibiotic spectrum can be narrowed as soon as the culture results become available. It is important to remember that a deep culture may miss up to two-thirds of the bacteria species present.83 Persisting signs of infection may be due to inappropriate antibiotics or undrained purulence or necrotic tissue.
For suspected osteomyelitis, obtain cultures of both the debrided osteomyelitic bone and the normal bone proximal to the area of debridement. It is very helpful to label the osteomyelitic bone culture as “dirty” and the normal proximal bone culture as “clean” so that the quality of the debridement can be assessed and the length of antibiotic treatment can be determined. If a wound is closed and the “clean” culture is showing no growth, then 1–2 weeks of antibiotic therapy is usually enough. On the other hand if the “clean” culture is positive for bacterial growth, then a re-resection of bone or a 6-week course of antibiotics may be necessary. A 6-week course of antibiotic therapy is no longer the accepted course of therapy if the involved infected bone has been surgically removed. When only healthy bone remains at the base of the wound, a 1-week course of appropriate antibiotics usually suffices. The exception to a 1-week course of antibiotics after closure is when the surgeon suspects that the bone left behind may still harbor osteomyelitis (e.g., calcaneus or tibia).84 In that case, a longer course of antibiotic therapy is needed. The appropriate antibiotic course is best determined and monitored by an infectious disease physician for treatment as well as for management of untoward side-effects.
Management
Trauma and crush injuries
Crush injuries or ischemic insults to the foot can elevate pressures within the myofascial compartments. When measuring the compartment pressures of the foot, it may be difficult to determine the compartment in which the pressure is actually being recorded. If clinical indications are present, a four compartment release should be performed.85
The medial approach to the foot, as advocated by Henry, allows decompression of all four compartments with a single incision.86 Mubarak and Hargens have advocated two longitudinal incisions on the dorsum of the foot through which the interosseous compartments may be decompressed (as in the hand).87 Whitesides anecdotally described compartment syndrome of the foot secondary to burns and direct trauma and recommended decompression using the medial approach of Henry.88
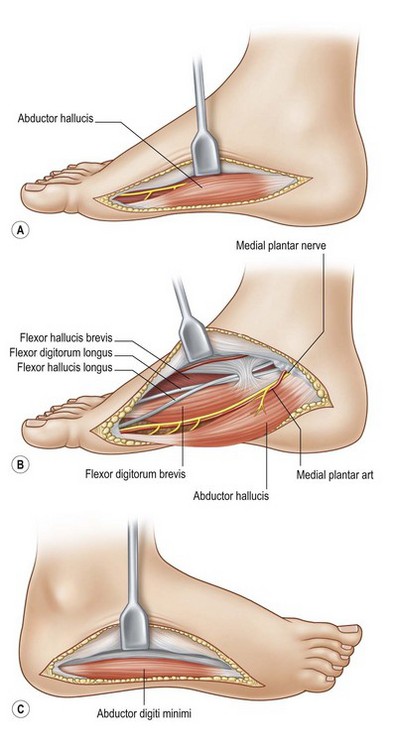
For compartment decompression, a curvilinear incision is made on the medial side of the foot, beginning at the first metatarsal head and extending to the heel. Divide both the skin and fascia and then reflect the flap plantarly to permit visualization of the abductor hallucis muscle and its tendon, thus decompressing the medial compartment. Retract the abductor hallucis towards the plantar surface and open the medial intermuscular septum, providing for release of the central compartment. The small toe compartment may be released next using a linear incision along the lateral aspect of the foot from the level of the fifth metatarsal head to the calcaneus. This approach adequately releases the fascia enveloping the small toe compartment. Decompression of the interosseous compartment is best achieved through two linear incisions on the foot dorsum overlying the second and fourth metatarsals. The fascia overlying the four interosseous spaces may be opened (Fig. 8.5).
Debridement of the chronic wound
Debriding a wound of necrotic tissue, foreign material, and bacteria/biofilm is essential as they ultimately impede the body’s attempt to heal by producing or stimulating the production of proteases, collagenases and elastases that overwhelm the local wound healing process.89 Bacteria produce their own wound inhibiting enzymes as well and consume many of the scarce local resources (oxygen, nutrition and building blocks) that are necessary for wound healing. Most of the offending bacteria reside within biofilm where they protect themselves from antibiotics and macrophages while inciting an inflammatory reaction around them.90 The biofilm tends to penetrate the base of the wound by spreading along the blood vessels that supply the surface in a process called “perivascular cuffing”. This penetration can be as deep as 4 mm suggesting that superficial scraping of the wound does little to get rid of the biofilm.
Steed reviewed the data of platelet-derived growth factor’s effect on the healing of chronic diabetic wounds91 and established level 2a evidence that wounds healed almost three fold more when the wound debridement was performed weekly rather than more sporadically. Two further level 2 studies support the critical role of debridement in wound healing.92,93
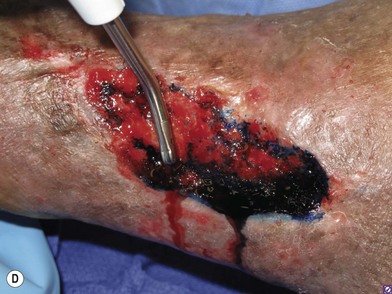
The principal debriding technique consists of removing the grossly contaminated or necrotic tissue en masse, while leaving a maximal amount of viable tissue behind. It is critical not to harm the underlying tissue as they will serve as the source for future wound healing. One has to use gentle wound handling techniques such as sharp dissection instead of cautery resection, skin hooks to retract the tissue, pinpoint use of the cautery to minimize the amount of charred tissue left behind, etc. One should avoid traumatizing techniques such as crushing the skin edges with forceps or clamps, burning tissue with electrocautery, or tying off large clumps of tissue with sutures.94 Surgical tools include a scalpel blade, mayo scissors, curettes, and rongeurs as well as power tools including a sagittal saw, a power burr, and a hydro-surgical debrider (Versa-Jet©, Smith & Nephew, Hull, UK). Debridement is performed as often as necessary until the wound is deemed clean and ready for reconstruction.
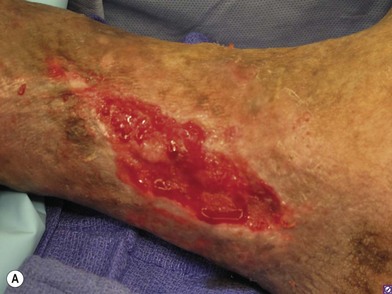
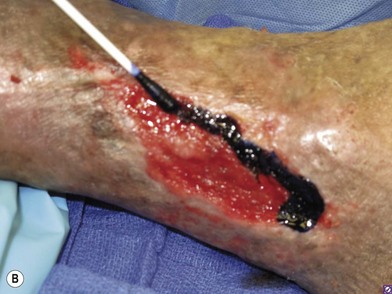
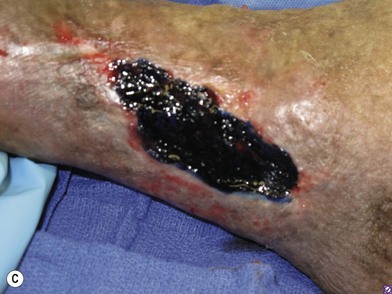
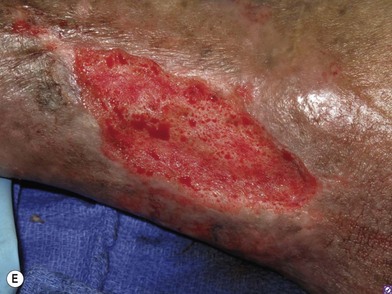
In order to be sure that the entire wound has been adequately debrided, three useful adjuncts are helpful. The first is to debride the wound until only normal tissue colors of red, white, and yellow remain at the base of the wound. The second is to first paint the wound base with blue dye using a cotton swab.95 After adequate debridement, there should be no blue dye left in the wound base. Finally, the edges of chronic wounds contain senescent fibroblast cells96 and an area of 3–5 mm of the tissues at the wound edge must be excised during the initial debridement to allow the healthy cells behind to help heal the wound (Fig. 8.6).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree