Foot and Ankle Reconstruction
Christopher E. Attinger
Mark W. Clemens
Functional restoration of the complex and efficient biomechanics of the foot and ankle is a challenge for the reconstructive surgeon. Trauma, tumor ablation, as well as changes in sensation, motor function, skeletal stability, blood supply, and immune status render the foot and ankle susceptible to breakdown. Inability to salvage the injured foot has traditionally led to amputation, carrying with it potentially dramatic morbid sequelae and a lifetime dependence on prosthetic devices. The relative 5-year mortality rate after major limb amputation in diabetics is greater than 50%, a startling figure when compared with mortality rates of lung cancer (86%), colon cancer (39%), and breast cancer (23%).
Successful foot and ankle reconstruction demands a team approach consisting of a vascular surgeon skilled in both endovascular and bypass techniques, a foot and ankle surgeon skilled in internal and external (Ilizarov) bone stabilization techniques, a soft-tissue surgeon for soft-tissue reconstruction, an infectious disease specialist, and a medical specialist to handle the comorbidities such as diabetes, hypertension, renal failure, and coronary artery disease.1 Surgical goals include a good local blood supply, debridement to a clean base, correction of any biomechanical abnormality, and nurturing the wound until it demonstrates signs of healing. Reconstruction can be accomplished by simple techniques 90% of the time and complex flap reconstruction in 10% of cases. This chapter focuses on the critical aspects of foot and ankle reconstruction, including anatomy, evaluation, diagnosis, and treatment with flap-based reconstructions.
ANATOMY
Vascular Anatomy
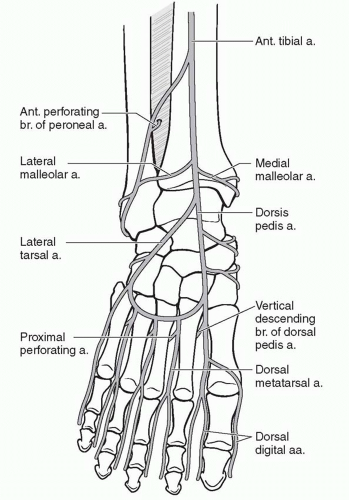
The foot and ankle consists of six angiosomes2: (1) the distal anterior tibial artery feeds the anterior ankle while its continuation, the dorsalis pedis artery, supplies the dorsum of the foot; (2) the calcaneal branch of the posterior tibial artery feeds the medial and plantar heel; (3) the calcaneal branch of the peroneal artery feeds the lateral and plantar heel; (4) the anterior perforating branch of the peroneal artery feeds the anterolateral ankle; (5) the medial plantar artery feeds the plantar instep; and (6) the lateral plantar artery feeds the lateral plantar mid- and forefoot (Figure 95.1). Note that the plantar heel receives dual blood supply from both the calcaneal branches of the posterior tibial and peroneal arteries. When the heel develops gangrene, this usually implies severe vascular disease involving both the peroneal and posterior tibial arteries.
Because the foot is an end organ, many arterial-arterial anastomoses provide a duplication of inflow. These arterial-arterial anastomoses (Figure 95.2) provide a margin of safety if one of the main arteries becomes occluded. At the ankle, the anterior perforating branch of the peroneal artery is connected to the anterior tibial artery via the lateral malleolar artery. At the Lisfranc joint, the dorsalis pedis artery dives into the first interspace to connect directly with the lateral plantar artery. This vascular loop is critical in determining the direction of flow within the anterior or posterior tibial arteries, which can be antegrade or retrograde or both. In addition, the plantar
and dorsal metatarsal arteries are linked to one another at the Lisfranc joint by proximal perforators and at the digital web spaces by distal perforators. Finally, the posterior tibial artery and peroneal artery are directly connected deep to the distal Achilles tendon by one to three connecting arteries. Using a Doppler ultrasound probe and selective occlusion, one can determine the patency of these connections as well as the direction of flow. This knowledge is critical in designing local flaps, pedicled flaps, and amputations.3
and dorsal metatarsal arteries are linked to one another at the Lisfranc joint by proximal perforators and at the digital web spaces by distal perforators. Finally, the posterior tibial artery and peroneal artery are directly connected deep to the distal Achilles tendon by one to three connecting arteries. Using a Doppler ultrasound probe and selective occlusion, one can determine the patency of these connections as well as the direction of flow. This knowledge is critical in designing local flaps, pedicled flaps, and amputations.3
Motor and Sensory Anatomy
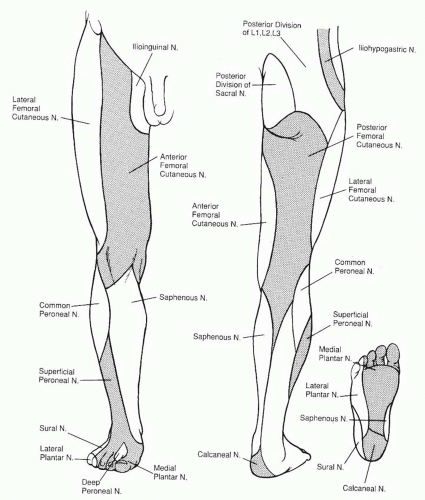
The sciatic nerve divides into the tibial and common peroneal nerves proximal to the popliteal fossa. The tibial nerve runs lateral to the popliteal vessels within the popliteal fossa, before entering the deep posterior compartment of the leg. The tibial nerve innervates muscles of the deep and superficial posterior compartments (except the gastrocnemius muscle) and trifurcates at the distal inner ankle, deep to the flexor retinaculum, into the calcaneal and medial plantar and lateral plantar nerves. These nerves provide the motor branches to the intrinsic muscles of the foot (except the extensor digitorum brevis [EDB] muscle). The common peroneal nerve passes around the lateral aspect of the fibular head before splitting into the superficial and deep branches. The deep peroneal nerve innervates the extensor muscles in the anterior compartment before exiting the extensor retinaculum to innervate the EDB muscle. The superficial peroneal branch innervates the everting peroneal muscles of the lateral compartment before it pierces the fascia to become subcutaneous and provide sensibility to the lateral lower leg and dorsum of the foot.
The sensory nerves to the foot and ankle (Figure 95.3) travel more superficially than the motor nerves, and their degree of function is a useful index to the localization of trauma. As mentioned, the superficial peroneal nerve (L4, L5, and S1) supplies the anterolateral skin in the upper third of the leg while descending within the lateral compartment. It becomes subcutaneous approximately 10 to 12 cm above the lateral ankle and travels anterior to the extensor retinaculum to supply the dorsum of the foot and skin of all the toes except the lateral side of the fifth toe (sural nerve) and the first web space (deep peroneal nerve). The deep peroneal nerve (L4, L5, and S1) exits the anterior compartment deep to the extensor retinaculum to supply and ankle and midfoot joints, sinus tarsi, and the first web space. The sural nerve (L5 and S1), derived from both the tibial and common peroneal nerves, descends distal to the popliteal fossa in the posterior aspect of the calf along the course of the lesser saphenous vein. It provides sensibility to the posterior and lateral skin of the leg’s distal third, prior to passing between the anterolateral border of the Achilles tendon and the lateral malleolus in order to supply the skin of the dorsolateral foot and fifth toe. The skin of the medial half of the lower leg and dorsomedial portion of the foot is innervated by the saphenous nerve (L5 and S1), a cutaneous branch of the femoral nerve. The dorsum of the foot has communicating branches between saphenous, sural, superficial, and deep peroneal nerves, and thus there is often an overlap in their respective terminal areas of innervation. As mentioned, the posterior tibial nerve at the distal portion of the tarsal tunnel
divides into three branches that supply the sole of the foot: the calcaneal branch (S1 and S2) supplies the medial aspect of the heel pad; the lateral plantar nerve (S1 and S2) supplies the lateral two thirds of the sole and the fifth and lateral fourth toes; the medial planter nerve (L4 and L5) supplies the medial one third of the sole and the first, second, third, and medial fourth toes. The medial and lateral plantar nerve can have an overlap in their respective zones with the saphenous and sural nerves, respectively.
divides into three branches that supply the sole of the foot: the calcaneal branch (S1 and S2) supplies the medial aspect of the heel pad; the lateral plantar nerve (S1 and S2) supplies the lateral two thirds of the sole and the fifth and lateral fourth toes; the medial planter nerve (L4 and L5) supplies the medial one third of the sole and the first, second, third, and medial fourth toes. The medial and lateral plantar nerve can have an overlap in their respective zones with the saphenous and sural nerves, respectively.
WOUND COMORBIDITIES
Diabetes
Approximately 24 million or 7.8% of all Americans have documented diabetes mellitus and 15% of them eventually develop a foot ulcer during their lifetime. Almost 15% of the health care budget of the United States goes toward management of diabetes, with 20% of hospitalizations and 25% of diabetic hospital days for the treatment of diabetic foot ulcers. Two thirds all the major amputations performed per year in the United States are performed in diabetics. Diabetics battle numerous complications related to their underlying disease, but none is more devastating, both psychologically and economically, than gangrene of an extremity with the associated risk of amputation.
Diabetic peripheral polyneuropathy is the major cause of diabetic foot wounds. More than 80% of diabetic foot ulcers arise in the setting of neuropathy. The neuropathy is a consequence of chronically elevated blood sugar that causes vascular and metabolic abnormalities. Elevated intra-neural concentrations of sorbitol, a glucose by-product, are thought to be one of the principal mechanisms for nerve damage. Further damage can result when the damaged nerve swells within anatomically tight spaces such as the tarsal tunnel. The combination of nerve swelling and tight anatomic compartments leads to the “double crush syndrome,” which may sometimes be partially reversed with nerve release surgery.4 Unregulated glucose levels elevate advanced glycosylated end product levels that may induce microvascular injury by cross-linking collagen molecules. Decreased insulin levels, along with altered levels of other neurotrophic peptides, may decrease maintenance or repair of nerve fibers. Other potential causative factors of peripheral neuropathy include altered fat metabolism, oxidative stress, and abnormal levels of vasoactive substances such as nitric oxide.
Hyperglycemia also affects the body’s ability to fight infection by diminishing the ability of polymorphonuclear leukocytes, macrophages, and lymphocytes to destroy bacteria. In addition, the diabetic’s ability to coat bacteria with antibiotics is diminished, which further helps shield bacteria from phagocytosis. As a result of this impaired immune state, diabetics are especially prone to Streptococcus and Staphylococcus skin infections. Deeper infections tend to be polymicrobial, with gram-positive cocci, gram-negative rods, and anaerobes present. Postoperative complication rates correlate directly with the level of postoperative hyperglycemia.
In patients with neuropathy, non-healing ulcers precede 80% to 95% of amputations. Despite attempts to decrease the number of amputations in the United States by various strategies from improving glucose control to more widespread screening exams for impaired sensibility, the number of amputations has continued to increase from 54,000 in 1990 to 65,700 in 2006. Arterial disease present in diabetic patients is usually located in the infra-popliteal region and significantly increases the risk of ulceration and possible amputation. It is present in greater than 50% of diabetic foot ulcers. So, while peripheral vascular disease is frequently present, peripheral neuropathy is the primary cause of foot wounds in the diabetic population.
Neuropathic Changes
The neuropathic changes in the diabetic feet are a result of the neuropathy in the motor, sensory, and autonomic nervous systems. The loss of pseudomotor function from autonomic neuropathy leads to anhydrosis and hyperkeratosis. Fissuring of the skin results and facilitates bacterial entry with subsequent infection. The lack of sensibility over bony prominences and between the toes often delays the detection of these small breaks in the skin.
Charcot deformities (neuroarthropathy) of the joints of the foot occur in 0.1% to 2.5% of the diabetic population. When present, the tarsometatarsal joints are involved in 30%; the metatarsophalangeal joints in 30%; the intertarsal joints in 24%; and the interphalangeal joints in 4% of the time. The explanation for these degenerative changes is widely debated. One possible etiology is “neurotraumatic,” i.e., joint collapse from damage that has accumulated because of insensitivity to pain. A more recent proposed etiology is due to osteopenia triggered by abnormalities in the RANK/RANK-ligand/osteoprotegerin system.
The process probably begins with a ligamentous soft-tissue injury accompanied by synovitis and effusion. In the absence of pain perception, continued use of the extremity exacerbates the inflammatory process. Eventually distention of the joint capsule leads to ligament distortion, resulting in joint instability. Further activity causes articular cartilage erosion, with debris being trapped within the synovium. These changes are often accompanied by loss of dorsiflexion of the foot due to the loss of Achilles tendon flexibility, adding further stress on the arch of the foot.5 This combination of changes can then cause a collapse of the medial longitudinal arch, altering the biomechanics of gait. The normal calcaneal pitch is distorted, which in turn causes severe strain to the ligaments that bind the metatarsal, cuneiform, navicular, and other small bones forming the long arch of the foot. Heterotopic bone formation and eburnation of load-bearing surfaces frequently result.
These degenerative changes overload specific parts of the foot rather than allowing the normal weight transition from heel to midfoot to forefoot. The increased focal stress leads to ulceration, infection, gangrene, and limb loss if the process is not halted or compensated for in its early stages. Diagnosis of a Charcot foot is often missed as it often presents as a swollen foot that is misdiagnosed as a sprain. Erythema may further confuse this presentation, leading to a misdiagnosis of cellulitis.
The motor component of the neuropathy further contributes to Charcot deformities as the intrinsic foot musculature atrophies and becomes fibrotic. The resulting metatarsophalangeal joint extension and interphalangeal joint flexion produce excessive pressure on the metatarsal heads and the ends of phalanges. The loss of both the transverse and longitudinal arches of the foot exacerbates the unfavorable weight distribution across the midfoot and metatarsal heads.
Ischemia
Atherosclerotic disease is a common cause of non-healing foot ulcers, especially in combination with diabetes. Hypercholesterolemia, hypertension, and tobacco use are additional risk factors for atherosclerosis. Other causes of ischemia in the foot include thromboangiitis obliterans (Buerger disease, generally seen in young smokers), vasculitis, and thromboembolic disease. The etiology of the ischemia requires accurate diagnosis before treatment is initiated.
When discussing revascularization plans with the vascular surgeon, it is important to consider within which angiosome the ulcer is located. Failure to revascularize the affected angiosome can lead to a 15% or greater limb loss rate despite a patent bypass. If the affected angiosome is directly revascularized, wound healing increases by 50% and the risk of major amputation decreases fourfold.6 For ulcers on the dorsum of
the ankle or foot, the anterior tibial artery or dorsalis pedis should be revascularized if possible. If the connection between the dorsalis pedis and the lateral plantar artery is intact, then a bypass to the posterior tibial artery is equally successful. For heel ulcers, revascularizing either the posterior tibial artery or peroneal artery is necessary. For mid- and forefoot plantar wounds, the posterior tibial artery should be chosen, although revascularizing the dorsalis pedis can be equally effective if the connection between the dorsalis pedis and the lateral plantar artery is intact. If the ideal vessel is not available, then revascularization should proceed with the understanding that there is a greater than 15% chance of failure.
the ankle or foot, the anterior tibial artery or dorsalis pedis should be revascularized if possible. If the connection between the dorsalis pedis and the lateral plantar artery is intact, then a bypass to the posterior tibial artery is equally successful. For heel ulcers, revascularizing either the posterior tibial artery or peroneal artery is necessary. For mid- and forefoot plantar wounds, the posterior tibial artery should be chosen, although revascularizing the dorsalis pedis can be equally effective if the connection between the dorsalis pedis and the lateral plantar artery is intact. If the ideal vessel is not available, then revascularization should proceed with the understanding that there is a greater than 15% chance of failure.
When the patient with significant peripheral vascular disease presents with gangrene, the timing of revascularization versus debridement is critical. If there is stable dry gangrene without cellulitis, then the revascularization should proceed promptly but nonurgently. If the patient presents with wet gangrene with or without cellulitis, the wound should immediately be debrided. Revascularization should then be performed on an urgent basis as progressive gangrene will occur without new blood flow. After revascularization, wound closure should be initiated only when the wound shows signs of healing with the appearance of new, healthy granulation tissue and neoepithelialization. It takes anywhere from 4 to 10 days after a bypass and up to 4 weeks after endovascular surgery for the wound to develop maximal benefit from the revascularization.
Connective Tissue Disorders
The connective tissue disorders (e.g., systemic lupus, rheumatoid arthritis, and scleroderma) cause difficult-to-treat recalcitrant vasculitis ulcers. These ulcers are frequently associated with Raynaud disease, which causes distal vasospasm and cutaneous ischemia. Treatment frequently requires immunosuppressive drugs such as steroids and immunosuppressive agents to control the autodestruction of tissue. Until the optimal immunosuppressive regimen is determined, the wound will not heal. The wound-retarding effects of steroids used in the immunosuppressive therapy are mitigated with oral vitamin A (10,000 U/d while the wound is open). The use of topical vitamin A is also effective. Close coordination with the rheumatologist is necessary in the management of these most difficult of wounds.
In addition, almost half of patients with vasculitic ulcers also suffer from a coagulopathy leading to a hypercoagulable state. The most frequent abnormalities involve antithrombin III, Leiden factor V, protein C, protein S, and homocysteine. Consequently, a coagulation blood panel is obtained on these patients and if abnormalities exist, they are treated with appropriate anticoagulants and/or medications by the hematologist.
The treatment of these ulcers is principally medical. Once the abnormalities are identified and corrected, wound-healing adjuncts can help in healing the wound. Cultured skin and hyperbaric oxygen can be used to stimulate the formation of a healthy granulation bed. Patience is required in treating these wounds as less than half go on to heal, which can take as long as 24 months.7
EVALUATION AND DIAGNOSIS OF THE WOUND
The etiology of foot and ankle wounds is often traumatic, with the underlying pathology complicating the healing process. Accompanying disease processes include infection, ischemia, neuropathy, venous hypertension, lymphatic obstruction, immunologic abnormality, hypercoagulability, vasospasm, neoplasm, self-induced wound, or any combination of the preceding. The most frequent systemic comorbidities include diabetes, peripheral vascular disease, venous hypertension, and connective tissue disorders.
Diagnostic Studies
Evaluation of the patient with a foot wound or ulcer begins with a history and physical examination. Important points in the history include etiology, duration and previous treatment of the wound(s), comorbid conditions (diabetes, peripheral vascular disease, venous insufficiency, atherosclerotic disease, autoimmune disorders, radiation, coagulopathy, etc.), current medications, allergies, and nutritional status. It is also important to assess the patient’s current and anticipated level of activity. Limb salvage is usually indicated if the patient uses the leg in any way (including simple transfers) and if medically tolerated and technically feasible. However, if the limb is not going to be used, then strong consideration should be given to performing a knee disarticulation or above-knee amputation to cure the problem and minimize the risk of recurrent breakdown.
When performing the physical examination, one should avoid the temptation to go right to the wound and examine the entire body. The wound examination includes measuring the wound (length, width, and depth) and assessment of the types of tissue involved (i.e., epithelium, dermis, subcutaneous tissue, fascia, tendon, joint capsule, and/or bone). The most accurate way of determining bone involvement is if one can directly feel bone with a metal probe, which correlates 85% of the time with the existence of osteomyelitis.6 Diabetic ulcers with an area >2 cm2 have a 90% chance of underlying osteomyelitis regardless of whether the bone is probed at the base of the wound. The levels of tissue necrosis and possible avenues of spread of infection via flexor or extensor tendons are then determined. If cellulitis is present, the border of the cellulitis is delineated with a marker and the date and time are noted. This permits the clinician to immediately monitor the progress of the initial treatment despite the lack of bacterial culture results.
The vascular supply to the foot is then examined. If pulses are palpable (dorsalis pedis or posterior tibial artery), there is usually adequate blood supply for wound healing. If one cannot palpate pulses, a Doppler should be used. The Doppler ultrasound probe also allows the surgeon to evaluate the nonpalpable anterior perforating branch and the calcaneal branch of the peroneal artery. It also helps determine the direction of flow along the major arteries of the foot to accurately assess local blood flow when designing a flap or amputation. A triphasic Doppler sound indicates excellent blood flow; a biphasic sound indicates adequate blood flow; and a monophasic sound warrants further investigation by the vascular surgeon. A monophasic tone does not necessarily reflect inadequate blood flow as it may reflect lack of vascular tone and absence of distal resistance.
If the pulses are non-palpable or monophasic, then noninvasive arterial Doppler studies are indicated. It is important to obtain PVRs (pulse volume recordings) at each level because arterial brachial indices are unreliable in patients with calcified vessels (30% of diabetics and all renal failure patients). Ischemia may be present if the PVR amplitude is <10 mm Hg. Obtaining arterial toe pressures yields further information because digital arteries are less likely to be calcified; if the toe pressure is <30 mm Hg, ischemia may be present. Tissue oxygen levels are also helpful in determining whether there is sufficient blood flow to the extremity. Tissue oxygen pressure levels <40 mm Hg suggest insufficient local blood flow to heal a wound. Skin perfusion pressure (Vasamed, Eden Prairie, MN) less than 50 mm Hg also indicates insufficient blood flow to heal. If the noninvasive tests suggest ischemia, an arterial imaging study is obtained to evaluate whether a vascular inflow and/or vascular outflow procedure is required. While bypass surgery remains the gold standard for revascularization, the less invasive endovascular techniques are very effective in treating stenosed or obstructed arteries by dilation, recanalization, or atherectomy with or without
stenting. Combined endovascular and bypass techniques are also effective.
stenting. Combined endovascular and bypass techniques are also effective.
Sensory examination is performed with a 5.07 Semmes-Weinstein filament that represents 10 g of pressure. If the patient cannot feel the filament, protective sensation is absent, leading to an increased risk of breakdown. In addition, one of the following should be used: vibration using 128-Hz tuning fork, pinprick sensation, or ankle reflexes. Motor function is assessed by looking at the resting position of the foot and the strength and active range of motion of the ankle, foot, and toes.
The bone architecture is evaluated by looking at whether the arch is stable, collapsed, or disjointed. Bone prominence can occur with collapsed midfoot bones (cuboid or navicular bone with Charcot destruction of the midfoot), osteophyte formation, or abnormal biomechanical forces (hallux valgus, hammer toe, etc.). An x-ray series of the foot is required (anteroposterior, oblique, and lateral). The views of the lateral foot should be weight bearing. Calcaneal, sesamoids, and metatarsal head views may be necessary if local pathology is suspected. It is important to remember that the x-ray appearance of osteomyelitis lags behind the clinical appearance by up to 3 weeks. A magnetic resonance imaging (MRI) scan can help with earlier detection of osteomyelitis, as well as with differentiation between osteomyelitis and Charcot collapse. In general, bone scans are of no value in evaluation of osteomyelitis when there is an ulcer present because the bone under an ulcer will show increased uptake, regardless of whether or not osteomyelitis is present. However, if proximal spread of osteomyelitis along a long bone is to be ruled out, then a negative bone scan can be very useful.
Finally, the Achilles tendon is evaluated. If the ankle cannot be dorsiflexed 10° to 15° beyond neutral, the Achilles tendon is tight and is placing excessive stress on the arch in the midfoot and on the plantar forefoot during gait. This needs to be addressed orthotically or surgically so as to avoid excessive pressure that could lead to Charcot collapse or forefoot plantar ulceration.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree