9 Flexor tendon injury and reconstruction
Synopsis
 Tendons transmit forces generated by muscles to move joints or to create action power. Flexor tendon injuries are common, but recovery of satisfactory function, particularly after injuries within the digital sheath, is sometimes difficult. Lacerated flexor tendons should be treated by primary surgical repair whenever possible.
Tendons transmit forces generated by muscles to move joints or to create action power. Flexor tendon injuries are common, but recovery of satisfactory function, particularly after injuries within the digital sheath, is sometimes difficult. Lacerated flexor tendons should be treated by primary surgical repair whenever possible.
 The current trend of end-to-end surgical tendon repairs is to use multistrand core sutures (four-strand repairs such as cruciate, double-Tsuge, Strickland, modified Savage, or six-strand repairs such as modified Savage, Tang).
The current trend of end-to-end surgical tendon repairs is to use multistrand core sutures (four-strand repairs such as cruciate, double-Tsuge, Strickland, modified Savage, or six-strand repairs such as modified Savage, Tang).
 In tendon repairs in the digital sheath area, a number of surgeons advocate that the A2 pulley can be released up to two-thirds of its length, and the A4 pulley can be entirely released when necessary and tendon repair is in the proximity of the pulley, given the integrity of the other pulleys. The release may reduce the resistance to tendon motion and the chance of repair ruptures. This technique is somewhat controversial.
In tendon repairs in the digital sheath area, a number of surgeons advocate that the A2 pulley can be released up to two-thirds of its length, and the A4 pulley can be entirely released when necessary and tendon repair is in the proximity of the pulley, given the integrity of the other pulleys. The release may reduce the resistance to tendon motion and the chance of repair ruptures. This technique is somewhat controversial.
 Postoperatively, early tendon mobilization should always be employed, except in children or in some rare instances; motion protocols vary greatly among different treatment centers.
Postoperatively, early tendon mobilization should always be employed, except in children or in some rare instances; motion protocols vary greatly among different treatment centers.
 Repair ruptures, adhesion formations, and finger joint stiffness are major complications of primary surgery.
Repair ruptures, adhesion formations, and finger joint stiffness are major complications of primary surgery.
 Combined use of multistrand core repairs, release of constricting pulley parts, and well-designed postoperative combined passive and active motion protocols – that do not overload, but sufficiently move the tendon – can help minimize adhesions, avoid repair ruptures, and restore optimal function.
Combined use of multistrand core repairs, release of constricting pulley parts, and well-designed postoperative combined passive and active motion protocols – that do not overload, but sufficiently move the tendon – can help minimize adhesions, avoid repair ruptures, and restore optimal function.
 Secondary surgeries include tenolysis, free tendon grafting, and staged tendon reconstruction. Tenolysis is indicated when restricting adhesions hamper tendon gliding and soft tissues and joint conditions of the hand are favorable. Free tendon grafting is a salvage operation for failed primary repairs, delayed treatment (>1 month) of an acute cut, or lengthy tendon defects. Staged reconstruction is indicated in case of extensive scar formation or multiple failed surgeries. Preservation or reconstruction of major annular pulleys is vital to restoring function of the digits during these secondary surgeries.
Secondary surgeries include tenolysis, free tendon grafting, and staged tendon reconstruction. Tenolysis is indicated when restricting adhesions hamper tendon gliding and soft tissues and joint conditions of the hand are favorable. Free tendon grafting is a salvage operation for failed primary repairs, delayed treatment (>1 month) of an acute cut, or lengthy tendon defects. Staged reconstruction is indicated in case of extensive scar formation or multiple failed surgeries. Preservation or reconstruction of major annular pulleys is vital to restoring function of the digits during these secondary surgeries.
 Closed ruptures of flexor tendons usually require surgical repairs.
Closed ruptures of flexor tendons usually require surgical repairs.
 The success of flexor tendon surgeries is very expertise-dependent. A thorough mastery of anatomy and meticulous surgical technique are requirements for satisfactory restoration of function.
The success of flexor tendon surgeries is very expertise-dependent. A thorough mastery of anatomy and meticulous surgical technique are requirements for satisfactory restoration of function.
Introduction
Difficulties in restoration of function of digital flexor tendons relate chiefly to the intricate anatomy of flexor tendon systems: the coexistence of superficialis and profundus tendons within a tight fibro-osseous tunnel. Frequent peritendinous adhesions jeopardize tendon gliding. Tendons within the synovial sheath (intrasynovial tendons) were once considered to lack the capacity for self-repair; therefore, invasion of adhesions from peritendinous tissues was believed to be a prerequisite in the tendon-healing process.1–4 As the concepts regarding tendon-healing biology evolved, tendon cells have proved capable of proliferating and of producing collagens to heal the tendons eventually.5–10 However, the tendon is innately low in cell density and growth factor activity, limiting its early healing strength.
In the early and middle 20th century, secondary tendon grafting dominated the repair of digital flexor tendons. During this period, tendon implants were developed for staged tendon reconstruction. However, as the practice of primary repairs prevailed in recent decades, the number of cases indicated for secondary tendon grafting or staged reconstruction decreased drastically. Primary repair of injured digital flexor tendons was pioneered by Verdan11 and Kleinert et al.12 in the 1960s and is the essential approach underlying current practice. Current primary repairs and inception of early tendon motion were based on the recognition of the intrinsic healing capacity of tendons in the 1970s and 1980s by Lundborg, Manske et al., and Gelberman et al.5–10
Nevertheless, despite the widespread use of primary repairs, surgical outcome remained unpredictable, and sometimes even disappointing. In the last two decades, major efforts were thus devoted to tackling this problem, with the goal of achieving consistently optimal outcome and minimizing repair ruptures and adhesions. In this regard, a number of multistrand core surgical repairs – such as the techniques of Savage, Strickland, cruciate, Lim-Tsai, or Tang13–19 – have been developed to replace weaker, conventional two-strand repairs. Subdivisions of zones 1 and 2 of digital flexor tendon systems were proposed by Moiemen and Elliot,20 and Tang,21 who offer precise nomenclature when recording the locations of tendon cuts, discussing treatment, and comparing outcome. Surgical procedures to release critical parts of the pulleys have been advocated by Tang22 and Kwai Ben and Elliot23 to decompress the tendon and free tendon motion. In the last few years, we witnessed reports in which repair ruptures were completely avoided, with recovery of excellent or good function in most cases.19,24,25 These recent reports represent remarkable steps towards satisfactory flexor tendon repairs and highlight the promise of predictable tendon repairs (Box 9.1).
Box 9.1
General tips for surgeons of flexor tendon repairs
• Repairing flexor tendons requires meticulous surgery built upon a thorough master of anatomy and biomechanics of the flexor tendon system. Surgeons should know the anatomy in detail, including the length of major pulleys, characteristic changes in the diameter of the sheath, and tendon gliding amplitude
• Primary repairs should be performed by experienced surgeons whenever possible, or if a less experienced surgeon has to be the operator, before surgery the surgeon must review the anatomy of the flexor tendon system, and understand every detail of the requirements of an optimal tendon repair
• The mastery of atraumatic techniques is essential for the operator. The outcome of the repair is very expertise-dependent: repair of tendons by an inexperienced surgeon is a frequent cause of tendon adhesions and poor function, thus should be avoided
• Conventional two-strand repairs are weak; stronger surgical repairs are preferable
• Complete closure of the tendon sheath is not a necessity. Venting of a part of sheath (<2.0 cm), including a critical portion of the pulleys, provides easy access to injured tendons, and decreases resistance to tendon gliding after surgery; this procedure does not lead to loss of digital function when other sheath parts are intact
• Surgeons should emphasize strong suture techniques and decreasing gap formation, which will lead to early active motion exercises and better outcomes
Historical perspective
Documentation and treatment of tendon injuries date back to antiquity. Hippocrates and other ancient physicians observed a slender white tubular structure entering skeletal muscle, and considered this to be a nerve. Galen, who lived in the Roman Empire in the second century, advised physicians not to repair tendons because it was believed this would result in pain, twitching, and convulsions of the limb. Over the following 15 centuries, surgeons were largely influenced by the teaching of Galen. The seminal challenge to Galen’s assertion did not occur until the mid 18th century when Von Haller showed that placing a suture in a canine Achilles tendon did not have detrimental effects.26
The first experimental investigation of the tendon-healing process was performed in 1767 by John Hunter, who recorded that the canine Achilles tendon healed by the formation of callus, similar to that seen in healing bone.26,27 However, specific studies of flexor tendon healing within the digital sheath were not started until about 150 years later, in the early 20th century. Around 1920, Salomon observed poor tendon healing of sutured canine flexor tendons.28 Salomon thus advocated leaving a defect in the tendon sheath to allow contact between the repaired tendon and the subcutaneous tissue.29 Bunnell and Garlock observed the frequent occurrence of restrictive adhesions at the flexor tendon laceration site.30–32 Bunnell coined the term “no man’s land” to describe the region where flexor tendons passed through the digital sheath and advised surgeons to be cautious when repairing tendons in this region. In 1940, Mason advised specific conditions for repairing lacerated digital flexor tendons, which included never repairing both tendons, wide excision of the overlying sheath, and adequately eliminating contaminates from the wound.33 Because of the generally unsatisfactory results of primary flexor tendon repairs, many surgeons preferred tendon grafting, despite the fact that a few surgeons had demonstrated that primary surgery was practicable at that time.
In the early half of the 20th century, repairing lacerated tendons within the digital sheath was predominantly by means of secondary tendon grafting. The first series of flexor tendon grafts in the hand was reported by Lexer in 1912.34 He used grafts to repair flexor tendons after rupture, old lacerations, infection, and cases with ischemic contracture. In 1916, Mayer published articles that have served as the basis of the present-day concepts of free tendon grafting.35–37 He emphasized the need for existing surgical techniques, direct juncture of the tendon to bone, the use of adequate muscle as a motor, and preserving peritenon around a graft to lessen adhesions. In 1918, Bunnell published a classic article on tendon grafting in which atraumatic technique, a bloodless field, perfect asepsis, and preservation of pulleys were emphasized.30 The surgical techniques of tendon grafting were subsequently modified by various leaders in the field, including Pulvertaft,38 Graham,39 Littler,40 Boyes,41 and Stark.42
In an effort to combat serious scarring in the tendon gliding bed in which conventional one-stage free tendon graft likely failed to restore tendon function, various materials have been implanted into the fingers to stimulate formation of a smoother tendon gliding bed. In 1936, Mayer inserted a celloidin tube into a seriously scarred tendon gliding bed, and after 4–6 weeks of implantation he then introduced a tendon graft.43 Mayer introduced the pseudosheath concept. In the 1950s Bassett and Carroll began working with flexible silicone rubber rods to build pseudosheaths in badly scarred fingers, adding passive motion to this procedure.44 This method was later refined into a two-stage tendon reconstruction by Hunter in the 1960s.45 Hunter and Salisbury46 also developed the active tendon implant. These implants and two-stage reconstruction procedures are still used currently, and are effective for patients with badly scarred digits and as a salvage procedure after other surgery attempts have failed.
It was not until the 1960s that reports of surgical techniques and outcomes of primary repair from Verdan11 and Kleinert et al.12 became the turning point in establishing the practice of primary repair of digital flexor tendons. The emerging popularity of primary tendon repair stimulated experimental studies to elucidate mechanisms of the healing process. In the 1970s and 1980s, Manske et al. demonstrated diffusion of nutrients through synovial fluid to be an effective source of nutrition of intrasynovial tendon, thus obviating the need for vascularization in the healing process.47–50 Matthews and Richards observed healing of lacerated rabbit flexor tendons within the intact digital sheath in the absence of adhesions.5,51–54 Lindsay et al. noted that both epitenon and endotenon cells proliferate and migrate to the laceration site, and subsequently bridge it.5,55–57 Lundborg et al. demonstrated healing of lacerated flexor tendon segments when placed within the synovial environment of the knee joint or in a synthetic membrane pouch placed in a subcutaneous pocket of the back of the rabbit.6,7 In the 1980s, Manske, Lesker, Gelberman, and Mass and others demonstrated healing of lacerated flexor tendon segments of different animals when placed in tissue culture in the complete absence of extrinsic cells.9,10,58–61
In the last three decades, major advances in the field of flexor tendon repairs were made with respect to the development of novel surgical repair techniques, accumulation of biomechanical information regarding tendon repairs or gliding mechanisms, modifications of postsurgical rehabilitation methods, and an emerging trend of exploring biological approaches to enhance tendon healing. In 1985, Savage13 published an influential experimental study that later stimulated a widespread interest in developing multistrand suture techniques to repair the tendon.62–74 The cruciate repair, a simple yet strong four-strand repair developed by McLarney et al.16 in the late 1990s, is used in many hand centers. In the same period, multistrand repair using Tsuge’s looped suture lines, as developed by Lim and Tsai, Tang (and its modification, M-Tang method),17–19,68 have been used by surgeons in hand centers in Asia, Europe, and North America. In 1995 and 1998, Tang22 and Kwai Ben and Elliot23 presented an anatomical study and clinical results of the release of a major flexor pulley (A2) to accommodate gliding of the repaired tendons, respectively. Elliot and Tang also proposed venting the A4 pulley when the tendon repairs are in the areas.19,23
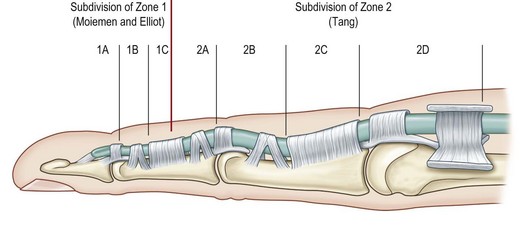
The flexor tendons were divided into five zones by Verdan in the 1960s.11 In the 1990s, subdivisions of zone 1 by Moiemen and Elliot20 and zone 2 by Tang21 were added to the existing zoning system, in order to describe the location of injuries and repairs more specifically. These zoning and subzoning systems provide hand surgeons with the nomenclature to document tendon injuries and outcomes, and discuss the principles of treatment.
In the past 30 years, biomechanics of the flexor tendon system has become a subject of intense interest for both surgeons and basic scientists. A large volume of information regarding tendon gliding and repair biomechanics has been obtained,74–122 including surgical repair techniques (mainly from the labs of Gelberman, Manske, Mass, McGrouther, Tang, Trumble, and Wolfe), gliding resistance of tendons within the sheath or against annular pulleys (Amadio and Tang), mechanics of the pulleys (Amadio, Mass, and Tang), postsurgical rehabilitation (Amadio and Boyer), and edema formation (Tang).
In recent years, investigations exploring molecular events in the tendon-healing process, tendon tissue engineering, and biological approaches to enhance the healing came to the center stage of basic science investigations. Chang et al. reported a series of investigations exploring the function of growth factors in tendon healing, molecular methods to prevent adhesions, and tendon tissue engineering.123–135 Tang and colleagues performed a series of studies of molecular events in tendon healing and gene therapy approaches to enhance strength or limit adhesions.136–146
A comprehensive history on the development of flexor tendon repairs has been covered in more detail in the reviews written by Verdan (1972),147 Manske (1988),148 Strickland (2000),149 Elliot (2002),150 Manske (2005),26 and Tang (2007).19
Basic science
Anatomy
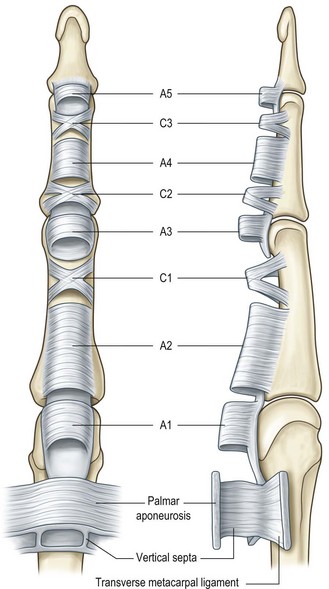
The digital flexor sheath consists of the synovial sheath and interwoven condensed fibrous bands (“pulleys”). The synovial sheath is a thin layer of continuous smooth paratenon covering the inner surface of the fibrous sheath, providing a smooth surface for tendon gliding and nutrition to the tendons. The pulley system of the digital flexor tendon is unique; it consists of annular pulleys (condensed, rigid, and heavier annular bands) and cruciate pulleys (filmy cruciform bands) (Fig. 9.1).151 There are five annular pulleys (A1–A5), three cruciate pulleys (C1– C3), and one palmar aponeurosis pulley.151,152 The A1, A3, and A5 pulleys originate from the palmar plates of the MCP, proximal (PIP) and distal interphalangeal (DIP) joints, and the A2 and A4 pulleys originate from the middle portion of the proximal and middle phalanges respectively. The broadest annular pulley is the A2 pulley, which covers the proximal two-thirds of the proximal phalanx and encompasses the bifurcation of the FDS tendon at its middle part. The A4 pulley is located at the middle third of the middle phalanx. The A2 and A4 pulleys are the largest among five annular pulleys and have the most important function. The annular pulleys maintain the anatomical paths of tendons close to bones and phalangeal joints, thus optimizing the mechanical efficiency of digital flexion. The more compressible cruciate pulleys allow for digital flexion to occur with condensation of the fibro-osseous sheath at the inner part of flexed fingers. This is called a “concertina effect.”
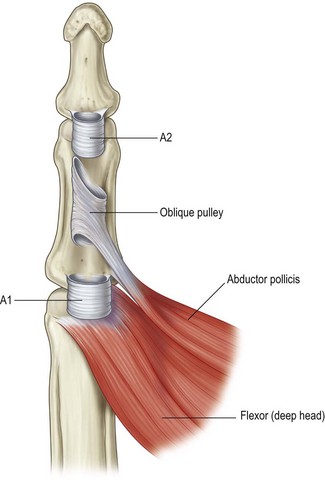
In the thumb, there are three pulleys (A1, oblique, and A2) with no cruciate pulleys (Fig. 9.2). The A1 and oblique pulleys are functionally important. The A1 pulley, 0.7–0.9 cm long, is located palmar to the MCP joint. The oblique pulley, 0.9–1.1 cm long, spans the middle and distal parts of the proximal phalanx. The A2 pulley is near the site of insertion of the FPL tendon, and is thin and 0.8–1.0 cm long.
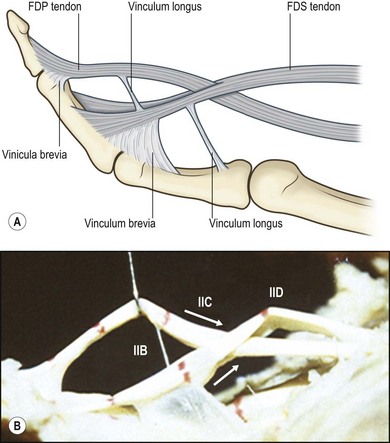
The FDP tendon has two vincula: a fan-like short vinculum and a cord-like long vinculum. The short vinculum is located at the insertion of the FDP tendon (Fig. 9.3). The long vinculum connects the FDP tendon through the short vinculum of the FDS tendon to the floor of the palmar surface of the phalanges. The FDS tendon also has two vincula: one connecting to the proximal phalanx, and another at the insertion of the FDS tendon. Vincula carry blood vessels to the dorsum of these tendons, providing limited nutrition. Tendon insertion sites to bones also carry vessels into tendons over a very short distance.
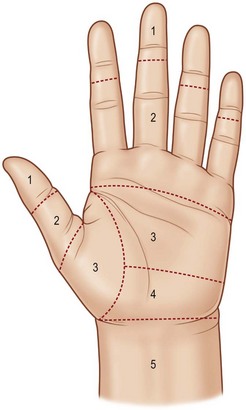
According to anatomical features, the flexor tendons in the hand and forearm are divided into five zones, which offer the fundamental nomenclature for flexor tendon anatomy and surgical repairs.153 In the 1990s, the most complex areas – flexor tendons in the digital sheath – were subdivided by Moiemen and Elliot,20 and by Tang.21 The zoning is described below, and its relation to the locations of pulleys is shown in Figures 9.4 and 9.5:
• Zone 1: from the insertion of the FDS tendon to the terminal insertion of the FDP tendon
• Zone 2: from the proximal reflection of the digital synovial sheath to the FDS insertion
• Zone 3: from the distal margin of the transverse carpal ligament to the digital synovial sheath
• Zone 4: area covered by the transverse carpal ligament
The subdivisions of zone 1 by Moiemen and Elliot are:
• 1A: the very distal FDP tendon (usually <1 cm), not possible to insert a core suture
• 1B: from zone 1A to distal margin of the A4 pulley
The subdivisions of zone 2 by Tang are:
Flexor tendon healing
Flexor tendons derive nutrition from both synovial and vascular sources. Flexor tendons outside the synovial sheath are supplied with a segmental vascular network through the paratenon, and the vascular supply plays an important role in the nutrition of these tendons. However, the tendons within the synovial sheath are mostly deprived of a vascular network. Only limited dorsal regions around vincular insertions are vascularized. A series of experiments by Manske et al. showed that intrasynovial flexor tendons are nourished by synovial fluid and that nutrition through vascular supplies is insignificant.47–50 While the general healing process of the tendon has long been recognized as having early inflammatory, middle collagen production, and late remodeling phases, the healing potential of the intrasynovial flexor tendon has been a subject of intense investigations and debate over several decades.1–10,52–61
Before the 1970s, it was widely accepted that the digital flexor tendon lacked intrinsic healing capacity.1–4 However, in subsequent decades, the intrinsic healing capacity of the tendon came to light in a series of elegant experimental studies. These experiments, by Matthews, Lundborg, Manske, Gelberman, and Mass and others, included observation of the repair process in lacerated flexor tendons within the synovial sheath, investigation of cellular activity in the lacerated tendon within the knee joint, detection of cellular activity, and the ability to produce matrix by in vitro tendon cultures.5–10,51–61 The work led to the well-supported conclusion that cells in the intrasynovial tendon can proliferate and participate in the healing process, making the tendon itself capable of healing without forming adhesions. This became the scientific basis of early postoperative tendon mobilization.
It is now agreed that intrasynovial flexor tendons can heal through two mechanisms – intrinsic and extrinsic. Intrinsic healing takes place through the proliferation of tenocytes and production of extracellular matrix by intrinsic cells. Growth of tissues or cell seeding from outside the tendon is extrinsic healing. The tendon’s intrinsic healing capacity is innately weak; extrinsic healing becomes dominant when intrinsic healing capacity is disabled (such as in the case of severe trauma to the tendon or peritendinous tissues) or under conditions (such as postsurgical immobilization) favoring extrinsic healing. Tendon healing exclusively through the intrinsic healing mechanism occurs only under a few experimental conditions.7–9 Clinically, the lacerated tendon heals through a combination of both intrinsic and extrinsic mechanisms, whose balance depends upon the condition of the tendon and surrounding tissues. Extrinsic healing may act on the tendon-healing process either by forming adhesions or seeding the extrinsic cells without adhesions to the laceration site. On the other hand, adhesions do not necessarily consist of extrinsic cells. Tenocytes may migrate out of the laceration site for a very limited distance to become part of adhesions. Conceptually, extrinsic healing does not equal adhesion formation. However, it is extrinsic healing in the form of restrictive adhesions that hampers tendon function.
Biomechanics of tendon repair and gliding
Forces generated during normal hand action range from 1 to 35 N, except tip pinch, according to in vivo measurements.154 Therefore, a surgically repaired tendon should be able to withstand a tension of at least 40 N during motion, with sufficient power to resist gap formation. The repair should be able to withstand cyclic loads under both linear and curvilinear load conditions. Laboratory tests have shown that conventional two-strand core repairs plus running peripheral sutures yield a maximal strength from 20 to 30 N93; this is lower than forces generated during normal hand actions and explains why some repairs are disrupted during postoperative motion exercise. Studies showed that failure forces of four-strand repairs are around or beyond 40 N16,65,94; six-strand repairs fail with loads over 50–60 N.13,71,85
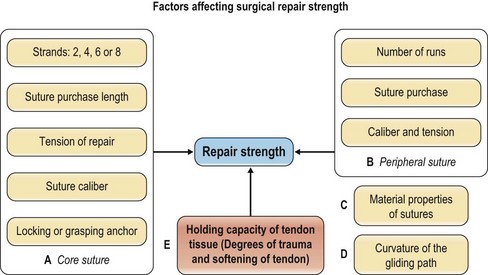
Many factors affect the strength of a surgical repair (Fig. 9.6): (1) the number of suture strands across the repair sites – strength is roughly proportional to the number of core sutures74–76,81–83,85,94–98; (2) the tension of repairs – this is most relevant to gap formation and stiffness of repairs19; (3) the core suture purchase84,86,88,94; (4) the types of tendon–suture junction – locking or grasping79,88,95,98; (5) the diameter of suture locks in the tendons – a small-diameter lock diminishes anchor power80,91; (6) the suture caliber (diameter)82,116,117; (7) the material properties of suture materials95; (8) the peripheral sutures155,156; (9) the curvature of tendon gliding paths – the repair strength decreases as tendon curvature increases89,90; and (10) above all, the holding capacity of a tendon, affected by varying degrees of trauma and posttraumatic tissue softening, plays a vital role in repair strength.
To achieve an optimal surgical repair, the factors outlined above must be considered and incorporated into repair design. A core suture purchase of at least 0.7–1.0 cm is necessary to generate maximal holding power, as recommended by Tang et al.86,93 and Cao et al.94 A locking tendon–suture junction is generally better than a grasping junction in terms of holding power. Diameter of the suture locks must reach or exceed 2 mm, according to Xie et al.91 Tan and Tang recommend a greater core suture purchase (>1.2 cm) and locking repairs for an obliquely cut tendon.86–88 Barrie et al.116 and Taras et al.117 greatly improved repair strength by increasing suture caliber. Clinically, the caliber of suture used in adults is either 3-0 or 4-0; sutures of 2-0 or greater are too large and rigid in the hand.
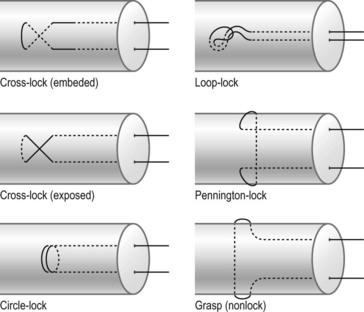
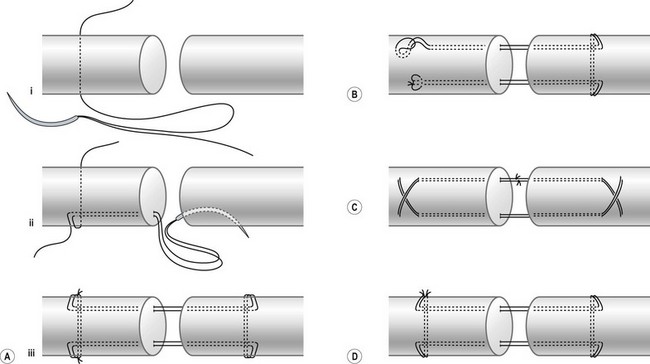
Tendon–suture junctions in surgical repairs are either grasping or locking; locking junctions vary greatly (Fig. 9.7). Grasping repairs are generally weaker than locking repairs. Among locking junctions, cross-locks provide identical strength to circle-locks.94 Exposed and embedded cross-locks create the same strength.92 With an identical number of suture strands across the tendon, different locking junctions result in minor differences in strength. Nevertheless, repairs with cross- or circle-locks appear slightly stronger than Kessler-type repairs with Pennington locks. Pennington locks provide a looser junction than cross- or circle-locks.
Peripheral sutures serve to “tidy up” the approximated tendon stumps; they may add strength to repairs as well. Deep-bite peripheral sutures increase repair strength.155 Increases in suture purchase or complex peripheral sutures, as typified by the Silfverskiöld method,64 increase overall strength. However, most surgeons choose to insert only simple peripheral stitches. Some surgeons even do not supplement peripheral stitches when multistrand core sutures have been used.25 In the presence of a strong multistrand core repair, peripheral sutures contribute little in terms of strength. In fact, to simplify repair maneuvers, multistrand core sutures (with or without a few peripheral sutures) may be sufficient.
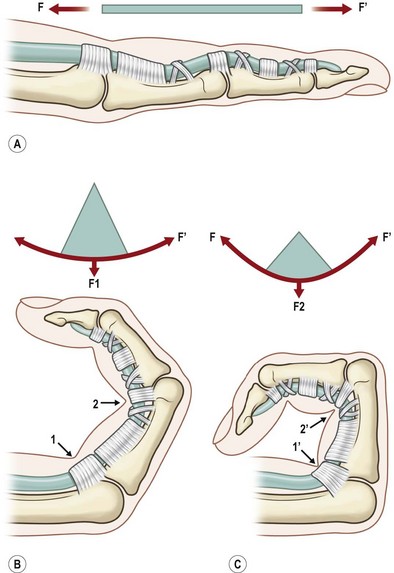
In addition to surgical factors, tendon curvature affects strength as well. Surgical repair in a tendon under a curvilinear load is weaker than that under a linear load; the repair strength decreases as the curvature increases.89,90 Mechanically, a tendon under linear tension is pulled without being bent, whereas a tendon under curvilinear tension is subjected to both linear pulling and bending forces. Therefore, the repair fails more easily in the flexed finger under curvilinear loads. When the finger moves to approach full flexion, a strongly bent tendon is particularly prone to fail (Fig. 9.8).
Annular pulleys are critical to the function of the digital flexor tendons. The pulleys keep the tendons’ course close to the phalanges for optimal mechanical efficiency of tendon excursion. Lengthy loss of the sheath and pulleys causes anterior displacement – bowstringing – of the flexor tendon during finger flexion. In fingers, the A2 and A4 pulleys are most critically located and functionally important. Preservation or reconstruction of the two pulleys is necessary in the absence of other pulleys or sheath. Nevertheless, given the presence of other pulleys and sheaths, the loss of any individual pulleys, including the A2 or A4 pulley, appears to result in few detrimental consequences. Both Tang22 and Tomiano et al.111,112 have shown that incision of the A2 pulley up to one-half or two-thirds of its length or of the entire A4 pulley results in no tendon bowstringing and little loss of digital flexion. In in vivo settings, incision of the A2 pulley decreases resistance to tendon motion and lessens the chance of repair failure.109,110 Loss of the A3 pulley alone has few consequences as well, but a lengthy sheath cut adjacent to the A3 pulley, containing C1 or C2, causes tendon bowstringing.107 Therefore, significant loss of sheath should be avoided to maintain tendon function; however, loss of a small portion (<2 cm in length) of the sheath and pulley, even including a part of the most critically located A2 pulley, has no substantial mechanical consequence.
Biological healing strength is a central issue underlying all tendon repairs. Urbaniak et al.,76 Kubota et al.,77 Aoki et al.78 and Boyer et al.114 have characterized the strengths of the healing flexor tendon using animal models. They found that strength either remained consistent or actually decreased somewhat over the initial few weeks after surgery.76–78 Decreases in strength, typically those in the second postsurgical week, are thought to be caused by softening of the tendon stumps, which lower the sutures’ holding power. Our investigations using a chicken model indicated that the strength of a healing tendon is steady during the initial 4 weeks, followed by a substantial increase (greater than threefold) in the fifth and sixth weeks; thereafter, the tendon heals strongly and is difficult to disrupt. The fifth and sixth weeks after surgery appear crucial to regaining strength. Accelerating healing, aiming to move this critical “strength gain” period to earlier weeks, is a current focus of research into molecular modulation of tendon healing.
Diagnosis/patient presentation
Careful attention to the patient’s history and the mechanism of injury can alert the surgeon to the extent of the tendon trauma and associated injuries. The natural resting posture of the wounded digits is important for evaluation. Complete lacerations of both FDP and FDS tendons are easily diagnosed when the affected fingers are seen in a relatively extended position with loss of active finger flexion at PIP and DIP joints. If the patient can actively flex the DIP joint while the motion of the PIP joint is blocked, no injuries or only partial injuries to the FDP tendon can be diagnosed (Fig. 9.9). To assess the continuity of the FDS tendon, the adjacent fingers are held in full extension by the examiner. If the patient cannot actively flex the PIP joint, the FDS tendon is completely severed (Fig. 9.10). Variations in the FDS tendons in the little finger are frequent. The FDS in 30–35% of the little fingers is connected with the FDS in the ring or middle fingers. Some little fingers (10–15%) are missing an FDS tendon. These patients have limited or no PIP flexion of the little finger during testing. Weakness during resisted finger flexion indicates a possible partial tendon cut. To test the FPL tendon, the thumb MCP joint is stabilized in a neutral position. The patient is asked to flex the IP joint. Loss of active flexion at the joint indicates complete severance of the FPL tendon.
Treatment/surgical techniques
Primary and delayed primary repairs
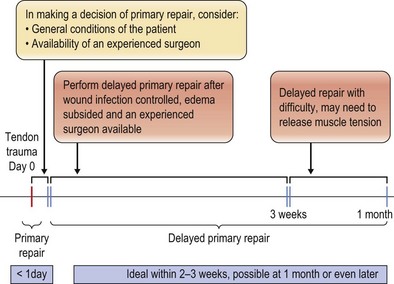
Whenever possible, acutely lacerated flexor tendons in the hand and forearm should be treated primarily or at the delayed primary stage. Primary tendon repair is the end-to-end repair performed immediately after wound cleaning and debridement, usually within 24 hours of trauma. Delayed primary repair is defined as repair performed within 3 or even 4 weeks after tendon lacerations. No clinical investigations actually validated the best time for primary repair. The ideal situation is that of a patient with digital flexor tendon lacerations brought into the clinic soon after injury; surgery begins within a few hours, and an experienced surgeon is readily available. The tendon injured in critical areas (such as zone 2) should not be repaired by an inexperienced surgeon. Rather, tendon repair can be delayed until an experienced surgeon is available. My preferred period of deliberate delay is 4–7 days, when the risk of infection can be properly addressed and edema has reduced substantially. Delay of the repair beyond 3–4 weeks may cause myostatic shortening of the muscle–tendon unit; for these late cases, lengthening the tendon within the muscles in the forearm can ease the tension (Fig. 9.11).157
Indications and contraindications
Primary or delayed primary end-to-end tendon repairs are mainly indicated in clean-cut tendon injuries with limited damage to peritendinous tissues. Neurovascular injury is not a contraindication for primary repairs. Loss of soft-tissue coverage over the tendon and the presence of fractures are borderline indications. Local defects in skin and subcutaneous tissues can be covered by flap transfer. A simple fracture limited to the phalangeal or metacarpal shaft can be securely fixed with screws or miniplates, and then tendons can be repaired. However, serious crush injuries, severe wound contamination, loss of extensive soft tissues, or extensive destruction of pulleys and tendon structures are contraindications for primary tendon repairs. Fractures involving multiple bones, particularly at different levels or not yielding stable internal fixation, are contraindications for primary tendon repairs (Box 9.2).
Surgical techniques
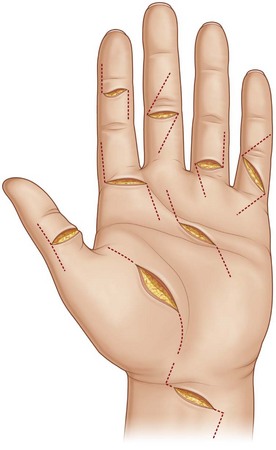
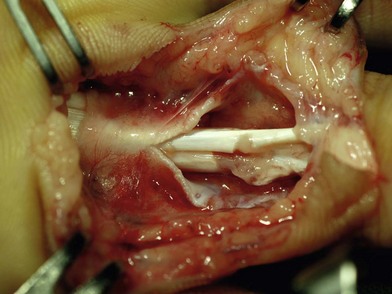
Brachial plexus block is usually sufficient; general anesthesia can also be used when associated injuries are severe. The hand and arm are scrubbed and draped. A tourniquet is placed on the upper arm. The wounds should be thoroughly debrided, devitalized tissues excised, and the wounds washed with antibiotic solution. The position of the fingers or hand is determined by levels of cuts in the tendons in relation to their superficial tissues. The hand is usually held by an assistant, so that it can be adjusted during surgery. Loupe magnification is advised for surgery. The tendons are exposed through zigzag skin incisions on the volar side of the fingers, e.g., Bruner’s incision, or a lateral incision. When the wounds are in the palm or forearm, incision by extending the wound opening is often necessary (Fig. 9.12).
Zone 1 injuries
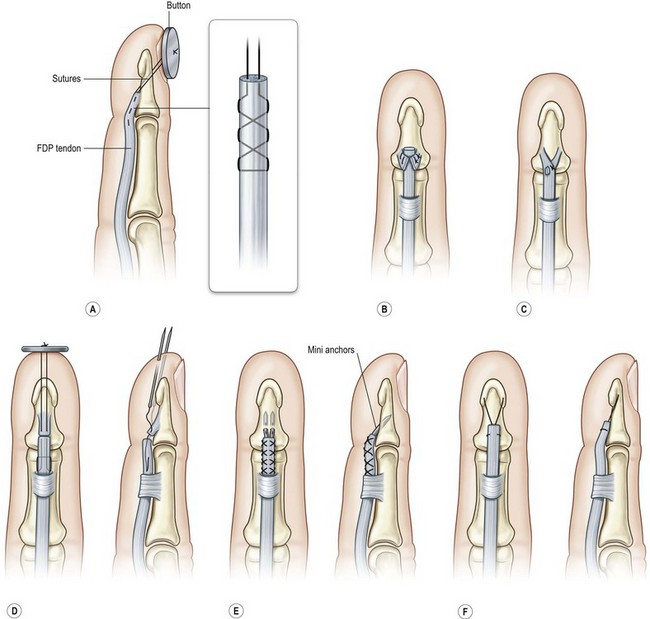
In this area, only the FDP tendon is located. When the tendon laceration is in the distal part of this zone (zone 1A and 1B), because the vincula connect to the proximal tendon to prevent retraction, both the proximal and distal ends can be easily found not far from the skin wound. When cut in zone 1C, the tendon may retract more proximally. For zone 1A injuries, the distal stump is usually too short for direct end-to-end repair. The proximal tendon end can be sutured with Bunnell or modified Becker suture with 3-0 polypropylene, and an osteoperiosteal flap is raised at the base of the distal phalanx (Fig. 9.13). The suture is led through an oblique drill hole, brought out through the nail, and tied over a button above the nail. To avoid passing the suture through the nail, the proximal tendons can be sutured to a fish-mouth opening in the distal tendon stump using reinforced suture repairs or minianchors (Fig. 9.13).156,157 Another method is to drill a transverse hole through the distal phalanx. After the tendon stump is sutured, the suture is led through the hole and tied to the other end, through an open approach or percutaneously.158,159 Injuries in zones 1B and 1C usually create tendon stumps of sufficient length for a direct surgical repair, which can be treated by methods similar to treatment in zone 2. Core tendon sutures, such as the modified Kessler, cruciate, modified Becker, or double Kessler repair, can be placed to the proximal end through a window opening in the proximal sheath. The proximal end is brought underneath the intact sheath between the wound and the proximal opening to approximate the distal end.
Zone 2 injuries
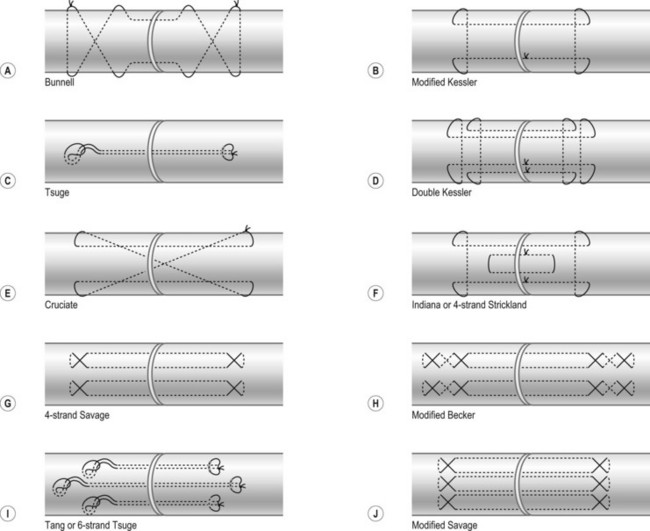
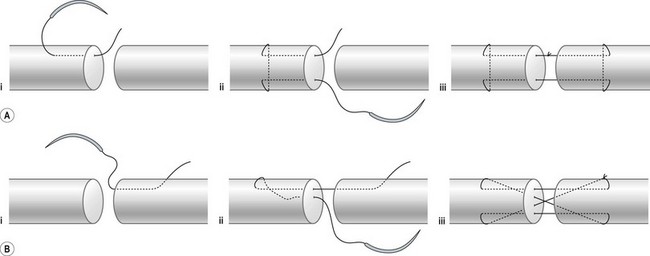
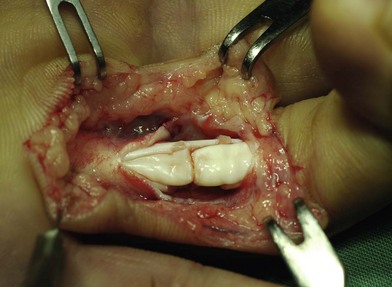
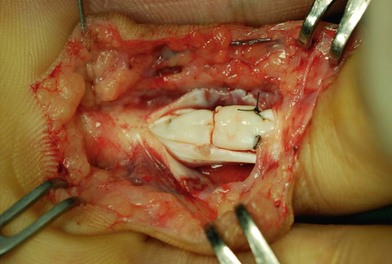
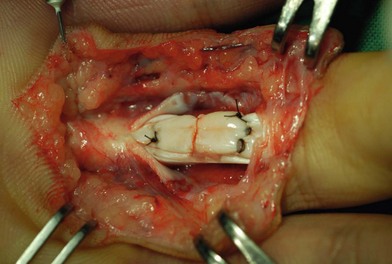
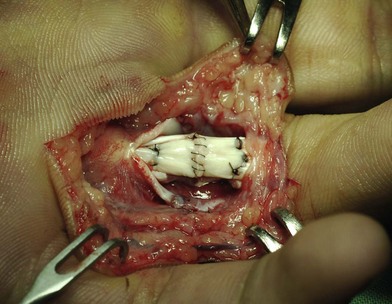
Surgical suture techniques vary among surgeons. Some core suture methods are shown in Figure 9.14. The modified Kessler and cruciate techniques are further shown in Figure 9.15. The Bunnell method is no longer popular for end-to-end repair. The two-strand modified Kessler method and Tsuge method are among the most widely used over the past 40 years. In the last 20 years, a number of multistrand repair techniques have emerged,14–19,65–69, including four-strand repairs such as cruciate, modified Savage, Strickland, and double Kessler; six-strand repairs such as Savage, Lim-Tsai, Tang, M-Tang; and eight-strand Winters–Gelberman methods. I prefer multistrand methods, typically four- or six-strand repair methods when repairing lacerated FDP tendons in zone 2.
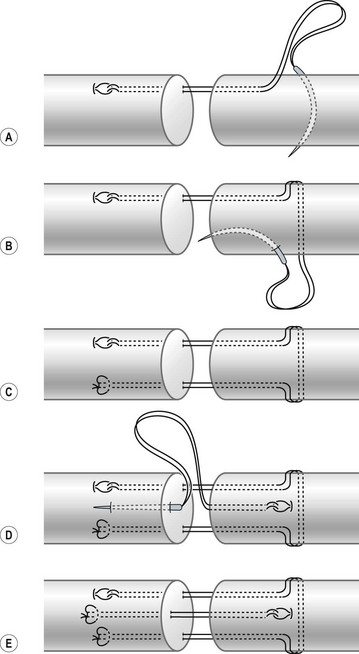
In my practice, I have used the double Tsuge method or six-strand methods in the past 20 years. In the last decade, my colleagues and I have started to use modifications of the original methods to repair tendons using fewer looped lines and knots, but maintaining suture strands and repair strength across the repair site identical to those of the original methods (Fig. 9.16). These methods are relatively easy and surgical repair strength is very reliable (Figs 9.17–9.23).
Using a needle carrying two separate suture lines, or with remaining pieces of the looped suture line after making the repairs described above, we can make a variety of four-strand Kessler-type repairs by introducing double sutures through one needle passage (Fig. 9.24). These techniques are used in my clinic as well as by my colleagues. We use these methods when repairing flatter tendons in some instances, such as the FDS cut proximal to bifurcation.72
Epitendinous stitches smooth the approximation of the tendon ends and resist gapping during tendon movement. Simple running peripheral, locking running peripheral, cross-stitch peripheral, and Halsted horizontal mattress sutures are among those most often used, with the first two more popular (Fig. 9.25). Some surgeons prefer “deep-bite” peripheral stitches to add strength to repairs.155 An epitendinous suture is usually added after the completion of core sutures, but it can also be added first.156 Peripheral stitches can be unnecessary given a strong multistrand core suture.25
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree