24 Flap classification and applications
Synopsis
 The use of flaps with an intact blood supply has revolutionized the field of plastic surgery. Today, the reconstructive surgeon faced with a soft tissue defect has a surplus of options.
The use of flaps with an intact blood supply has revolutionized the field of plastic surgery. Today, the reconstructive surgeon faced with a soft tissue defect has a surplus of options.
 The muscle flap, the musculocutaneous flap, fasciocutaneous flap, perforator flap and the various techniques of microvascular composite tissue transplantation have made possible major advances in the field of plastic surgery.
The muscle flap, the musculocutaneous flap, fasciocutaneous flap, perforator flap and the various techniques of microvascular composite tissue transplantation have made possible major advances in the field of plastic surgery.
 By applying a precise knowledge of the anatomy of skin, muscle, bone and fascia in planning reconstructive procedures, the surgeon has the ability to restore form and function in congenital and acquired defects in most topographic regions.
By applying a precise knowledge of the anatomy of skin, muscle, bone and fascia in planning reconstructive procedures, the surgeon has the ability to restore form and function in congenital and acquired defects in most topographic regions.
 Modifications and refinements in flap design offer considerable variety and versatility in the techniques available for use in reconstructive surgery.
Modifications and refinements in flap design offer considerable variety and versatility in the techniques available for use in reconstructive surgery.
 By applying the principles of flap design and technique, it is possible to simplify the approach to the surgical defect.
By applying the principles of flap design and technique, it is possible to simplify the approach to the surgical defect.
 Soft-tissue coverage, form and function are the three most important factors in determining a successful outcome.
Soft-tissue coverage, form and function are the three most important factors in determining a successful outcome.
 Through careful analysis of each individual surgical defect, the most appropriate method of reconstruction can be selected. This chapter reviews flap classification and gives examples of their applications.
Through careful analysis of each individual surgical defect, the most appropriate method of reconstruction can be selected. This chapter reviews flap classification and gives examples of their applications.
History
The use of flaps for reconstructive plastic surgery dates back to 600 bc, when the earliest recorded application of pedicled flaps for nasal reconstruction is attributed to Sushruta Samhita (translated by Bhishagratna in 1916).1 The earliest flaps centered on the head and neck as well as the lower extremity because wounds in these regions failed to heal by secondary intention. The initial flaps used would now be considered random-pattern flaps, as they were not based on a specific blood supply and were used without an understanding of how and why they survived. Tagliacozzi used a distally-based arm flap in a two-staged procedure.2 His work was published in Venice in 1597. Much of this knowledge was forgotten until the 19th century, when the English surgeon Carpue successfully used forehead flaps to reconstruct the noses of two officers.3 The publication of Rhinoplastik by Von Graefe’s in 1818, further advanced the use of these techniques.4 Attention in the early 20th century remained focused on tubed random flaps. It was found that the only way to increase survival of these flaps was to perform a surgical delay. A German anatomist, Carl Manchot, demonstrated the concept of anatomic skin territories supplied by consistent vessels in Die Hautarterien des Menschlichen Korpers, published in 1889.5 Tansini described the skin island of the latissimus dorsi musculocutaneous flap in 1906.6 Davis, crediting Manchot, demonstrated axial and pedicled muscle and fascial flaps, as well as composite flaps in 1919.7 McGregor introduced the temporalis flap, which allowed midface and lower face coverage without the donor site deformity associated with the previously popular forehead flap.8 Coverage of the lower third of the face as well as of the oral and esophageal defects was accomplished by Bakamjian with the use of the deltopectoral flap.9 The availability of the forehead, temporalis, and deltopectoral flaps changed the approach to head and neck cancer extirpative surgery with an emphasis on immediate reconstruction.
The muscle flap for lower extremity reconstruction was initially described by Stark for coverage of debridement sites for osteomyelitis.10 Unfortunately, this went unnoticed until Ger recognized that the leg muscles are a source of well-vascularized tissue for leg coverage.11 Although Owens, in 1955, used a compound flap consisting of the sternocleidomastoid muscle with overlying skin for head and neck reconstruction, the concept of musculocutaneous perforating vessels providing a cutaneous territory for superficial muscles was first reported by Orticochea in 1972.12 Shortly thereafter, surgeons made significant contributions toward definition of flaps, expansion, and use of muscle and musculocutaneous flaps in reconstructive surgery. The contributions included the concept of cutaneous territory of superficial muscles13; anatomy of the muscles including specific arcs of rotation14,15; applications of muscle and musculocutaneous flaps for breast, chest, extremity, and head and neck reconstruction; and microsurgical transplantation.16–21 In 1981, Ponten recognized the input of septocutaneous perforating vessels to the overlying skin circulation.22 On the basis of this observation, the concept of fasciocutaneous flaps was introduced. Like the muscle flap, the septocutaneous flap was initially described in the lower extremity, but the principle of the fasciocutaneous flap, based on muscle, fascia, and associated cutaneous territories, was rapidly applied to all body regions (Table 24.1).
Table 24.1 Timeline of the development of flap surgery
| 600 bc | Sushruta Samhita1 | Pedicle flaps in the face and forehead for nasal reconstruction |
| 1597 | Tagliacozzi2 | Nasal reconstruction by tubed pedicle flap from arm; described “delay” of pedicle flap |
| 1896 | Tansini23 | Latissimus dorsi musculocutaneous flap for breast reconstruction (post-mastectomy) |
| 1920 | Gillies24 | Tubed pedicle flap |
| 1946 | Stark10 | Muscle flaps for osteomyelitis |
| 1955 | Owens25 | Compound neck flap |
| 1963 | McGregor8 | Temporalis flap |
| 1965 | Bakamjian26 | Deltopectoral flap |
| 1971 | Ger11 | Lower extremity musculocutaneous flap |
| 1972 | McGregor and Jackson27 | Groin flap |
| 1972 | Orticochea12 | Musculocutaneous flaps |
| 1977 | McCraw et al.13 | Musculocutaneous territories |
| 1981 | Mathes and Nahai15 | Classification of muscle flaps based on vascular anatomy |
| 1981 | Ponten22 | Fasciocutaneous flaps |
Starting in the late 1970s, a proliferation of innovative techniques reported in the surgical literature throughout the world defined new anatomic data and applications of the muscle, musculocutaneous, fascial, and fasciocutaneous flaps. These advances in flap definition and application have changed the entire approach to plastic surgery. With the isolation of the vascular pedicles to muscle- and fascia-based flaps, microsurgical transplantation is selected when the ideal flap is outside of a traditional and safe arc of rotation to the recipient site. With the definition of musculocutaneous and fasciocutaneous territories, an expander may be used to enlarge flap dimensions and still ensure direct closure of the donor site. The reconstructive ladder, introduced in 1982, was appropriate for use in choosing reconstructive methods that ensure safety in soft-tissue reconstruction.28 Now, ideal form and function can be achieved by complex procedures without compromising safety. Furthermore, donor site deformity is frequently avoided because the flap can be precisely tailored to fit the defect. In 1997, the reconstructive triangle was introduced.29 The surgeon may choose the transposition flap, microsurgical transplantation, or tissue expansion with the goals of achieving form and function at the recipient site, avoiding donor site deformity, and providing safety throughout the reconstructive endeavor. The more recent introduction of perforator flaps has expanded our options even more and brought us to the era of free style flaps. The following information traces the development of the flap systems, defines the vascular anatomy, and provides an overview of flap classifications and applications.
Flap classification
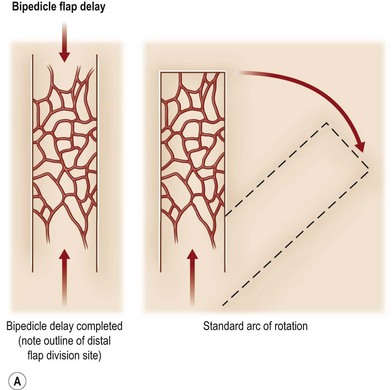
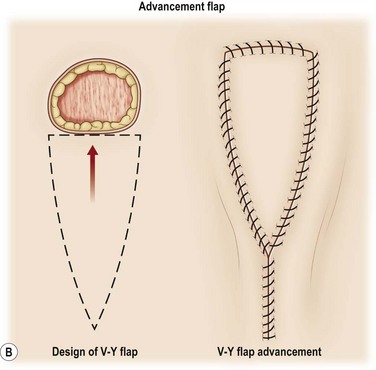
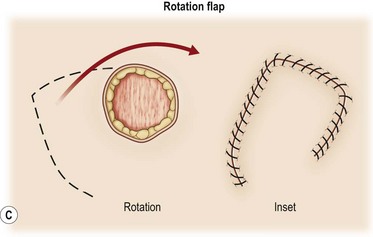
A flap consists of tissue that is mobilized on the basis of its vascular anatomy. Flaps can be composed of skin, skin and fascia, skin and muscle or skin, muscle and bone. Because the circulation to the tissue to be mobilized is crucial for flap survival, the development of flap techniques has depended on defining the vascular anatomy of the skin and underlying soft tissue. An early concept of vascular anatomy as it pertained to flap surgery was the thought that skin circulation was based on the longitudinal subdermal plexus. A random pattern flap based on this subdermal plexus was designed to allow elevation of a rectangular-shaped flap of skin and subcutaneous tissue with a length: width ratio in the range of 2–1.5 : 1. Milton subsequently disproved the concept of length-to-width ratios.30 Although limited in its reach, the random pattern flap can be elevated and rotated to provide viable skin and subcutaneous tissue to cover an adjacent wound. Common flaps based on the subdermal plexus include the bipedicle flap, advancement flaps (i.e., V–Y), and rotation or transposition flaps (Fig. 24.1).
Other restrictions of random flaps include the limited arc of rotation, the proximity of the flap to the wound and the associated zone of injury and decreased bacterial resistance.31 Given the vascular limitations of the random pattern flap, investigators attempted different means by which to maximize the potential area of a flap, which led to the concept of flap delay. Although the delay procedure has been used for several hundred years, it was not until the early 1900s that the concept was recognized. Blair introduced the term delayed transfer in 1921.32 In the 16th century Tagliacozzi delayed his upper arm flaps by making parallel incisions through the skin and subcutaneous tissue overlying the biceps muscle. In 1965, using the pig model, Milton investigated the effectiveness of four different methods of delaying a flap. He found that in developing a bipedicled flap, the best form of delay was by making two incisions and undermining the skin between the incisions.33 The goal of a delayed flap is to enhance flap circulation, ensuring flap survival after advancement, transposition, or transplantation to a defect site. Flap delay may be used to increase circulation to the muscle or fascia or to enhance vascular connections to the overlying cutaneous territory or adjacent structures to be included during flap elevations (tendon, fascia, and bone). Although delay may be accomplished by biochemical means to improve flap perfusion, currently the most effective method to ensure delay is surgical manipulation of the flap. To date, no pharmacologic method has surpassed the reproducibility and the degree to which surgical delay protects against flap necrosis.34 There are two theories that describe the potential mechanism by which the delay phenomenon prevents skin necrosis. The first is that delay acclimatizes the flap to ischemia (tolerance), permitting it to survive with less blood flow than would normally be required. This theory suggests that vascular delay cause adaptive metabolic changes at a cellular level within the tissue.35 The second theory is that delay improves vascularity by increasing flow through preexisting vessels, reorganizing the pattern of blood flow to more ischemic areas.36,37 On the basis of experimental data, it appears that both of these mechanisms, either directly or indirectly, contribute to the beneficial effects of surgical delay. Regardless of the underlying mechanisms, most experimental work on surgical delay demonstrates changes at the microcirculatory level.38,39
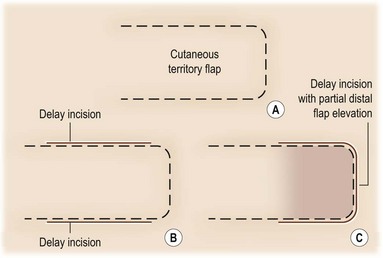
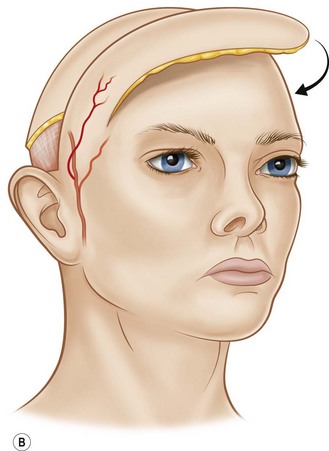
The technical aspects of standard surgical delay to enhance circulation are straightforward. The flap cutaneous territory is outlined and incisions are made through all or part of the border of a planned cutaneous territory (Fig. 24.2). Minimal to partial flap undermining is performed. The incisions are then closed. The flap is then elevated after 10–14 days. It has been shown that after 1 week, the blood flow into the area of delay reaches a maximum.40
Strategic pedicle delay is accomplished by making incisions at the border of the planned flap cutaneous territory. The dissection is either deep to muscle or fascia, depending on the flap type, to reach pedicles entering the flap territory. These pedicles are divided and the incision is closed. Second-stage flap elevation is performed after a 2-week period. Effectiveness of strategic delay based on ligation of the dominant vascular pedicle was initially demonstrated in the converse model of the gracilis musculocutaneous flap.21 This type of delay is usually advocated in patients with risk factors for flap ischemia (i.e., smoking history, obesity, radiation therapy, abdominal scar). An example of an indication for surgical delay would be a patient who is to undergo breast reconstruction with a TRAM flap. It has been shown that high-risk patients benefit from surgical delay.41 In addition, it has been shown that delay potentially reduces the incidence of abdominal wall complications.42 New techniques for strategic delay are designed to minimize incision length and procedure-related morbidity. Endoscopic techniques allow access and division of pedicles with minimal incisions. Interventional radiology techniques may also be used to occlude dominant or secondary pedicles to the flap territory to enhance perfusion to the remaining flap pedicles.
The delay phenomenon may result in part from a sympatholytic state that results from cutting the sympathetic innervation to the vasculature and the subsequent vasodilatation. Drugs that block vasoconstriction or those that vasodilatate may be of theoretic value. Attempts have been made to stimulate the delay phenomenon pharmacologically by manipulating the autonomic nervous system.43 Although pharmacologic delay is of theoretical importance, additional studies need to be done to prove its effectiveness.
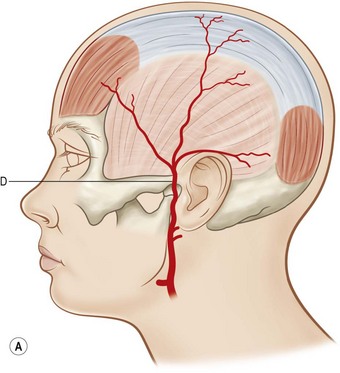
The erroneous concept that skin circulation is based on a longitudinal vascular network independent of deeper structures delayed the progress of flap discovery. A few isolated areas with direct cutaneous vessels allowed flap elevation without the 2 : 1 length-to-width ratio restriction (e.g., median forehead flap). However, the need for longer flaps without a requisite delay procedure resulted in the identification of flaps with specific vascular territories based on the course of the superficial vascular pedicles with an axial alignment (Fig. 24.3). Axial flaps based on this concept include the lateral forehead (superficial temporal artery), deltopectoral (internal mammary branches), superficial groin (superficial circumflex iliac artery), and dorsal foot (dorsalis pedis artery) flaps. The development of these axial flaps has had a tremendous impact, particularly in the head and neck and upper extremity reconstruction.
The longer, nondelayed axial flaps made immediate reconstruction of defects possible in the head and neck, groin, and upper extremity spurred the search for new flaps based on consistent vascular pedicles in the trunk and extremities. Muscle was soon identified as a source of tissue that could be detached from its normal origin or insertion and transposed as a flap based on its major (dominant) vascular supply. Further analysis of skin circulation revealed that there were important musculocutaneous perforator vessels supplying the overlying skin, altering the approach to flap design.12–14 This eventually led to the concept that muscles and fascia have distinct vascular pedicles. The success of muscle flaps in reconstructive surgery is based on reliable blood supply. With knowledge of the location and subsequent preservation of the vascular pedicles to muscles, every muscle may be rotated as a flap.
Muscle and musculocutaneous flaps
1. The regional source of the pedicle entering the muscle
2. The number and size of the pedicle
3. The location of the pedicle with respect to the muscle’s origin and insertion
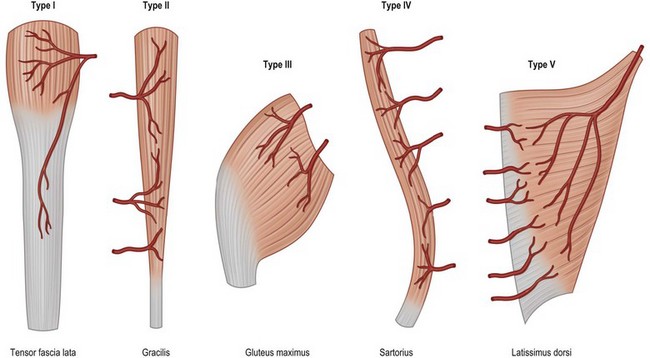
This classification system enables the surgeon to categorize the various muscle and musculocutaneous flaps into distinctly different, clinically applicable groups based on the vascular anatomy. There are five different vascular patterns by which the various muscles are categorized (Fig. 24.4).15
Type I: one vascular pedicle
Type I muscles are supplied by a single vascular pedicle (Table 24.2).
Table 24.2 Type I vascular pattern muscles
Type II: dominant vascular pedicle and minor pedicle
Type II muscles are supplied by both a dominant and minor vascular pedicle. The larger dominant vascular pedicle will usually sustain circulation to these muscles after the elevation of the flap when the minor pedicles are divided. This is the most common pattern of circulation observed in human muscle (Table 24.3).
Table 24.3 Type II vascular pattern muscles
Get Clinical Tree app for offline access 
|