Fig. 30.1
Multipotent adipose-derived mesenchymal stem cells (ADSCs)
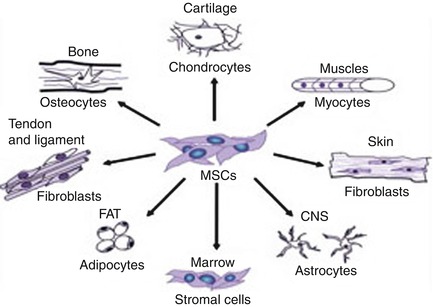
Fig. 30.2
MSCs and their main differentiation
MSCs are phenotypically heterogeneous in terms of morphology, physiology, and surface antigen expression. No specific marker has yet been identified for them, but they seem to express a large number of adhesion molecules, extracellular matrix proteins, cytokines, and growth factor receptors. As stem cells, they are characterized by their ability to self-renew and differentiate toward multiple cell lineages [26]; a further way of identifying possible MSC populations is by inducing them to differentiate into bone [27], fat, and cartilage in vitro. According to the literature, MSCs from different sources have been successfully differentiated into osteoblasts, chondrocytes [28], adipocytes [29], fibroblasts, myoblasts and cardiomyocytes [30, 31], hepatocytes, tenocytes, and even neurons [32]. Some MSCs are already used for orthopedic, cardiac, and neuro-repair [33], while others are being investigated.
It is known that MSCs are attracted on injured or pathological tissues by means of still unknown mechanisms but that possibly involve chemokines and their receptors [34], as well as adhesion molecules. MSCs are capable of expressing genes of embryonic origin, adhesion molecules, extracellular matrix proteins, collagen, fibronectin, etc.; they secrete several interleukins, such as IL-7, IL-8, IL-10, and IL-11, as well as growth factors stimulating hematopoiesis as SCF (stem cell factor), G-CSF (granulocyte-colony stimulating factor), and countless cytokines [35]. They also support the in vivo homing of HSCs: they secrete stromal-derived factor (SDF-1) which regulates the migration of hematopoietic stem cells expressing the receptor CXCR-4 (CD84) in the bone marrow [36]. Studies concerning the genetic profile of MSCs show a different pattern of gene expression, suggesting the existence of functionally different subpopulations depending on the source.
Clinical applications of MSCs in regenerative and aesthetic medicine seem to be due mainly to their paracrine effect, which means the production of chemical messengers able to spread and change the physiology of surrounding cells and homeostasis of their microenvironment. The most current evidence would be that the strong biological activity (secretion of soluble markers after systemic infusion of MSCs) would supply the lack of typical local engraftment of these cells and that would mean two things:
1.
The source of MSCs can be important in determining the biological activity because different tissues may originate MSCs with different profiles of cytokine expression.
2.
The isolation and expansion in culture conditions (seed density, culture medium, serum, added cytokines) may considerably influence the gene expression, reprogramming the bioactivity of these cells.
30.5 Stem Cell Niche Therapy Using Adipose Tissue
Adipose-derived stem cell niche refers to the complex relationships that ADSCs establish with all of the physiological or ectopic factors contributing to determine their identity and fate. The phenotypical identity of any cell and its reaction to a given stimulus are, therefore, not only determined by the genetic or epigenetic equipment of the cell but also depend on the specific niche context in which it resides. A change in niche parameters or the transplantation of cells into other niches can have a considerable effect on cell physiology and alter its properties.
Research has shown that the signal controlling the embryonic origin of ADSCs and their differentiation in adult adipose tissue include the same pathway as that involved in the homeostasis of other adult and embryonic stem cell populations [37, 38], and their complex mechanism can have different effects depending on the concentration, stage of differentiation, and extrinsic niche factors, such as cell-matrix and cell-cell interactions, presence of vasculature, and type of innervation. Despite extensive research about the intracellular signaling involved in ADSC homeostasis and differentiation, little is known about the role of cell-cell and cell-matrix interactions [39, 40].
Zannettino et al. [41] suggested that ADSCs reside in perivascular niches, which prompts the idea that perivascular structures (cells and extracellular matrix) may provide signals that balance the maintenance of ADSCs in an undifferentiated state and their commitment to differentiation. It has recently been shown that in vitro culture significantly alters the transcriptional phenotype of ADSCs. Freshly isolated stromal vascular fractions (SVF) express hematopoietic markers (CD34) that are lost within a few days of culturing [42]. In line with previously published findings [43], the increased expression of mesenchymal stem cell-associated markers in cultures of human and murine ADSCs has been documented, whereas the longer-term loss of markers of undifferentiated status, such as undifferentiated transcription factor (UTF-1), indicates a shift toward differentiation. Prolonged culturing also significantly downregulated various isoforms of procollagen, matrix metallopeptidases, and inflammatory cytokines, thus indicating adaptation to the artificial environment. Under extreme conditions, it has even been reported that in vitro long-term culturing can induce the neoplastic transformation of ADSCs [44], although it is still unclear whether these changes are reversible or how they may affect the therapeutic potential of the cell.
However, ADSCs can be used in many clinical applications (particularly in the fields of plastic and reconstructive surgery) by means of transplantation without removing them from their fat niche. In addition to molecular and cell biology, the dynamic and regenerative nature of fat grafts has been established in various areas of plastic surgery, and clinical practice has shown that long-term outcomes of fat grafting can include rejuvenation of skin texture [45]. Ultrastructural studies of centrifuged fat have revealed that mature adipocytes show interruptions in their cytoplasmic membrane and various degrees of degeneration including cell necrosis, but the stromal vascular fraction (SVF) appears to be well preserved [46].
Rigotti et al. [47] compared the ultrastructure of mammary radiolesions before, and at different times after fat grafting, and found clear signs of regeneration, with evidence of new adipocytes. Before transplantation, almost all of the adipocytes were seriously damaged by centrifugation, and in the radio-damaged recipient tissue, there were neither mature adipocytes nor differentiating preadipocytes. Reasonable interpretations of these findings are that the differentiating preadipocytes observed after treatment were either committed adipocyte originally embedded within the transplanted fat or endogenous locally present committed adipocyte, activated by the ectopically transplanted fat. The massive survival of ADSCs in fat transplants, despite liposuction and centrifugation procedures, strongly supports the first hypothesis, but the second may be equally valid as it assumes that transplanted fat enriched with ADSCs by centrifugation can behave as an atypical ectopic niche that direct tissue regeneration by modulating endogenous tissue resources.
The term “atypical ectopic niche” could be used as a common paradigm of stem cell-based therapies. It is based on the idea of a “bystander” mechanism in which the stem cells ectopically transplanted into a generic lesion (radiolesion, myocardial infarction, cerebral stroke, etc.) do not replace tissue-specific cells by direct differentiation, but locally form an atypical stem cell niche that suppresses inflammation, promotes neo-angiogenesis, and favors the activation of endogenous stem cell precursors by releasing trophic factors, such as cytokines, proangiogenic molecules, and growth factors. Therefore, adipose tissue can be considered an ideal “biologic product” [48]. The role of this essential component of the stromal fraction of the lipoaspirates argues for a regenerative theory based on angiogenesis imbued by various growth factors, not yet fully identified, released from stem cells. Thus, in addition to the traditional understanding that fat is a high-energy reservoir involved in homeostasis for its ability to bind large amounts of fluids and in the maintenance of thermoregulation, it becomes apparent that it is also a regenerative organ providing the basis for soft tissue regeneration.
According to the sub-mentioned theories, nowadays ADSCs have become one of the most popular adult stem cell populations for research in soft tissue engineering and regenerative medicine applications. Compared with other stem cell sources, they offer several advantages including an abundant autologous source, minor invasive harvesting (liposuction), significant proliferative capacity in culture, and multi-lineage potential. To harvest the adipose tissue, a liposuction procedure is less invasive than bone-marrow aspiration, and the technique produces less patient discomfort and donor site morbidity. Small amounts of adipose tissue yields approximately 5 × 103 stem cells, which is 500-fold greater than the number of MSCs in 1 g of bone marrow [49].
Numerous preclinical studies have been pursued, with early clinical data appearing in the literature. Fat as a living, free graft does more than just fill the area into which it is placed. Grafted fat affects the tissue into which it is placed in many ways for the life of the patient. It can improve the quality of aged and scarred skin and heal radiation damage and chronic ulcers. Just how grafted fat causes these changes remains unanswered. We know that fat can perform amazing feats in a glass tube and in some animal models, but we have little insight into what happens to fat when it is grafted from one part of the human body to another part. The focus of the near future should be research to explain and expand on future clinical successes.
In addition to the outstanding results of lipotransfer for volume restoration, surgeons have become increasingly interested in the apparent rejuvenative effects on the skin itself. Coleman has noted improved skin quality, with softening of wrinkles, decreased pore size, and improved pigmentation during the first year posttreatment. In fact, analysis of clinical results following both reconstructive and aesthetic surgery reveals that after one or several repeated fat grafting procedures, the trophicity and quality of the recipient site is improved. These changes develop over several months following the fat grafting procedure, with a variable expression, as improvements in skin texture, skin suppleness, skin color, and/or scar quality.
Topographical skin analysis systems such as the recently developed VISIA system (Canfield Imaging Systems) may determine whether the effect from fat grafting is an effective skin rejuvenation technique in comparison to chemical peels and laser resurfacing. Aside from effects on normal, aged skin, when fat has been grafted beneath depressed scars, there was improvement not only in the depression but also in the character of the scar itself, with an apparent transformation to normal-appearing skin. Other authors have reported an adverse range of improvements in soft tissue conditions, including radiation damage, breast capsular contracture, damaged vocal cords, and chronic ulceration, as well as regrowth of calvarial bone. While many of the exact mechanisms for these effects remain to be described, what seems to be at the center of these changes is the presence of adipose-derived stem cells (ASCs).
30.6 Benefits of Stem Cells in a Fat Transfer
1.
Grow new blood vessels to nourish the fat.
2.
Release anti-inflammatory agents to aid healing.
3.
Generate and release growth factors that support graft survival.
4.
Improve skin tightening and rejuvenation.
5.
No risk of allergic or adverse reaction.
6.
A cutting-edge option used around the world today.
7.
The therapeutic target is the reconstruction of autologous niches, by simply moving stem cell niches from one connective tissue in which they are abundant into another in which they are scarce.
30.7 Research Directions
The possible application of ADSCs for tissue repair and healing has recently been researched. It has been experienced that
1.
Local injection of ADSCs into skin ulcers induces rapid healing with less scarring in a rat model.
2.
Topical administration of ADSCs around the site of primary sciatic nerve repair in rats improves the functional restoration of the sciatic nerve, a result that has been confirmed by gait analysis, electroneurography, and histology.
These therapeutic models may be applicable to clinical situations in which the environment for wound healing is compromised by inadequate blood supply and severely scarred tissue. Gimble et al. have shown that ASCs delivered into an injured or diseased tissue may secrete cytokines and growth factors that stimulate recovery in a paracrine manner. ADSCs modulate the stem cell niche of the host by stimulating the recruitment of endogenous stem cells to the site and promoting their differentiation along the required lineage pathway. In a similar way, ADSCs could provide antioxidants, free radical scavengers at an ischemic site: as a result, toxic substances released into the local environment would be removed, thereby promoting recovery of the surviving cells [23]. Further preclinical and clinical studies need to be performed so that ADSC-based therapies fulfill expectations and can be successfully used to treat disorders for which the present medical and surgical therapies are either ineffective or impractical.
30.8 Aging and Rejuvenation
Atrophy is the defining feature of aging: it also represents a primary challenge in the evolving quest to find the best approach for rejuvenating aging faces and bodies. Rejuvenation is not about suspension or sagging: it is all about augmentation in order to restore youthful fullness and contour in a natural and aesthetic fashion. One major goal of rejuvenation procedures is to rebalance fat and restore harmony to the face. This can be accomplished by microliposuction of the fatty “hills” and fat transfer to the sunken “valleys.” Restoration of homogeneity to the facial structure reduces the sharp shadows associated with aging.
Augmentation represents the past and the future of rejuvenation. It also holds the key to possible new developments such as tissue repair. Examination of the results over time reveals promising posttreatment changes in the quality of the patient’s skin after the placement of grafted fatty tissue under it. In addition to restoration of youthful contours, intrinsic qualities of skin texture, elasticity, and color return to a more youthful state for an extended period of time. Stem cells have been shown to be present in significant quantities in harvested fatty tissue. Perhaps they are mending the sun-damaged, aging epithelium.
Sagging of the aging face may occur mostly as a result of changes in the fat compartments that are coincident with chronological aging. Localized overabundance of fat may weigh down the tissue. Conversely, an area devoid of fat resembles a deflated balloon by inducing the downward displacement of facial skin. Inelastic recoil due to photodamage compounds this effect. If altered fat distribution underlies differences between the young and the old face, a new model for the youthful countenance might arise.
A young face has a smooth, ample distribution of fat. It resembles a continuous, “gently rolling plain” because the fat is evenly distributed. There is a forward projection with facial arcs highlighting specific areas and causing minimal shadow. In contrast, the aging face has “hills and valleys” producing deep shadows and irregular highlights. In thin individuals, these “hills” of fat may be minor, but in most middle-aged adults, the hills occur in a strip down the central face from mid-cheek to jowl, along the nasolabial and labiomental folds. Fat pocketing can also be seen suborbitally on the lateral zygoma, submentally, and along the neck platysmal bands. Since body fat rises with age, so does facial fat. “Valleys,” in contrast, occur periorbitally and periorally, in the malar, buccal, and temporal areas and on the far lateral cheek.
Fat loss manifests around the mandible and throughout the forehead and scalp. The most common areas of the face which are treated with autologous fat grafts include the nasolabial groove, marionette lines, midface, and lips. Autologous fat performs best in the midface area considering the longevity compared to other more mobile areas such as the lips and marionette grooves.
30.9 Structural Fat Grafting
30.9.1 Harvesting
The authors (ADG) started using autologous fat transfer or fat grafting procedures in the last 3 years to provide lasting natural structure and contour changes in the face. Various methods for harvesting and refining autologous fat grafts have been described.
One of the standard procedures, the Coleman technique [50, 51], is based on manual aspiration with syringes to reduce the negative pressure and the centrifugation of the graft. It is also known as structural fat grafting or lipostructure. Various studies have shown that this technique causes little damage to the cells and have demonstrated survival of the tissue transferred. Typically, the authors use 3–10 mL syringes with 16-gauge needles or cannulas to harvest subcutaneous extra fat from the abdomen, lateral or inner thighs, gluteal areas, or medial knees. Current studies have not indicated increased viability from any donor site, so harvesting sites are chosen for ease of accessibility and to improve the patient’s body contours. They are accessed through incisions placed or increases, previous scars, stretch marks, or hirsute areas whenever possible. Anesthetic solution is infiltrated into the sites of fatty tissue removal. During harvesting, we take care to minimize mechanical trauma to the fragile parcels of fat (Fig. 30.3).
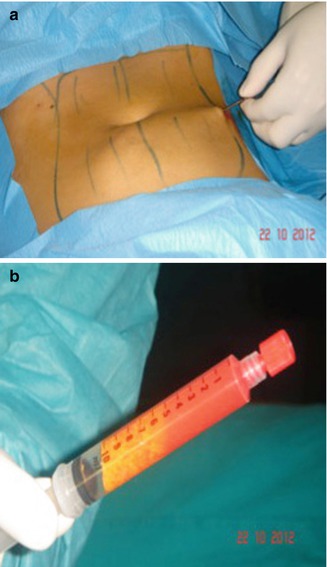

Fig. 30.3
(a, b) Fat samples from the abdomen before centrifugation
30.9.2 Refinement and Transfer
The suctioned fat is specially processed by centrifuging to separate the oil and fluids to isolate the living fat. The centrifugation also concentrates the fatty tissue, associated stem cells, and growth factors from the unwanted fluids, oil, and nonliving components. Our recommended centrifugation speed is 3,000 rpm (1,500 × g) for 3 min. Larger centrifuges can create significantly more gravitational force at 3,000 rpm than the smaller centrifuges commonly used in offices (Fig. 30.4). With the centrifugation, three layers are formed in the syringe: the top one is oily and consists essentially of spilled material traumatized by fat cells; the lower one is the most dense between the three and is formed by blood and saline solution; and finally the middle one contains fat living cells that will be infiltrated in the area to be corrected (Fig. 30.5).
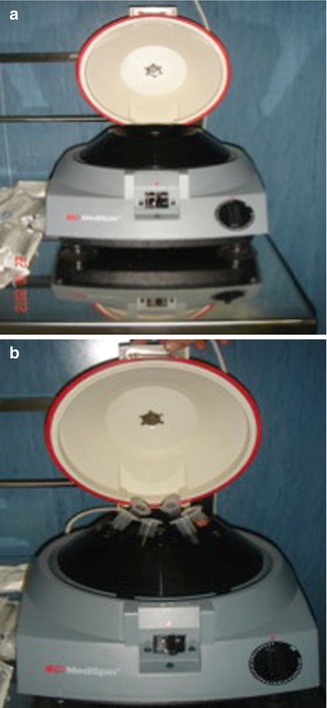
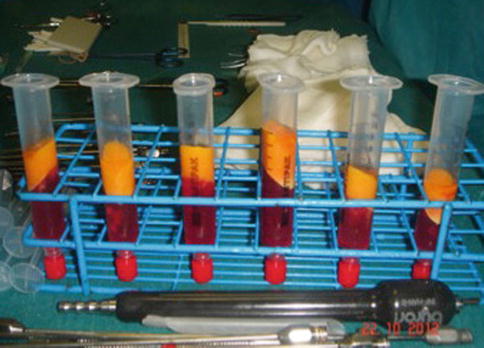

Fig. 30.4
(a, b) Centrifuge machine (central rotor and sleeves)

Fig. 30.5
Fat samples from the abdomen post-centrifugation
Both the upper and the lower layers are removed, respectively, using absorbent wicks (Codman neuropads) with the plunger and, after exerting a slight pressure, in the syringe remains the only intermediate layer. It is necessary to isolate the adipocytes to be transplanted, as much as possible, in order to decrease the adipocytes to be transplanted in order to decrease inflammatory response after replanting; if many cellular debris are present in the recipient site, they develop an intense inflammatory reaction with the activation of cells of flogosis that are recalled to clean the affected area. Some studies also point to the importance of the fourth layer (so-called pellets), in the superficial part of the lipoaspirates, as a key source of mesenchymal stem cells and endothelial cells (Fig. 30.6).
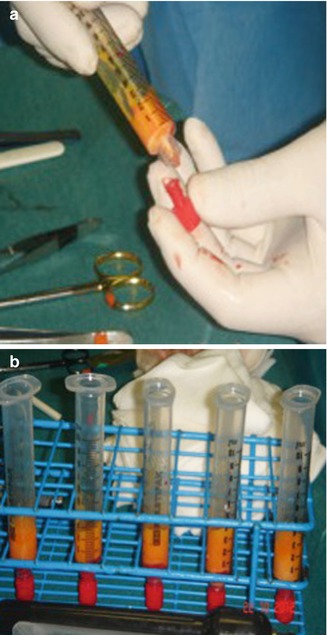

Fig. 30.6
(a, b) Removal of the upper and the lower layers in every sample of centrifuged fat
Then, the refined fat is transferred into a 1 or 3 mL Luer-Lock syringe and we carefully place it into the areas to be treated. The transfer of the harvested fat to 1 mL syringes and blunt cannulas of various curves and sizes offers precise placement of small 0.1 mL aliquots of fat. The placement cannulas are of a much smaller gauge than the harvesting cannulas: the gauge used can range from 14 gauge to 21 gauge. (The most useful size of cannula for placement in the face is 17 gauge.) The most suitable lengths for use in facial procedures are from 5 to 9 cm. However, larger-bore cannulas can be used for corporal fat grafting, and smaller gauges may be appropriate in areas such as in the lower eyelids. For various situations in the face and body, cannulas with different tip shapes, diameters, lengths, and curves can be used. Using blunt cannulas allows placement of the fat parcels in a more stable or precise placement and less traumatic manner. However, less blunt cannulas may give us more control for placement in the immediate subdermal plane, in fibrous tissue, and in scars. A cannula with pointed or sharp elements can be used to free up adhesions (Fig. 30.7).
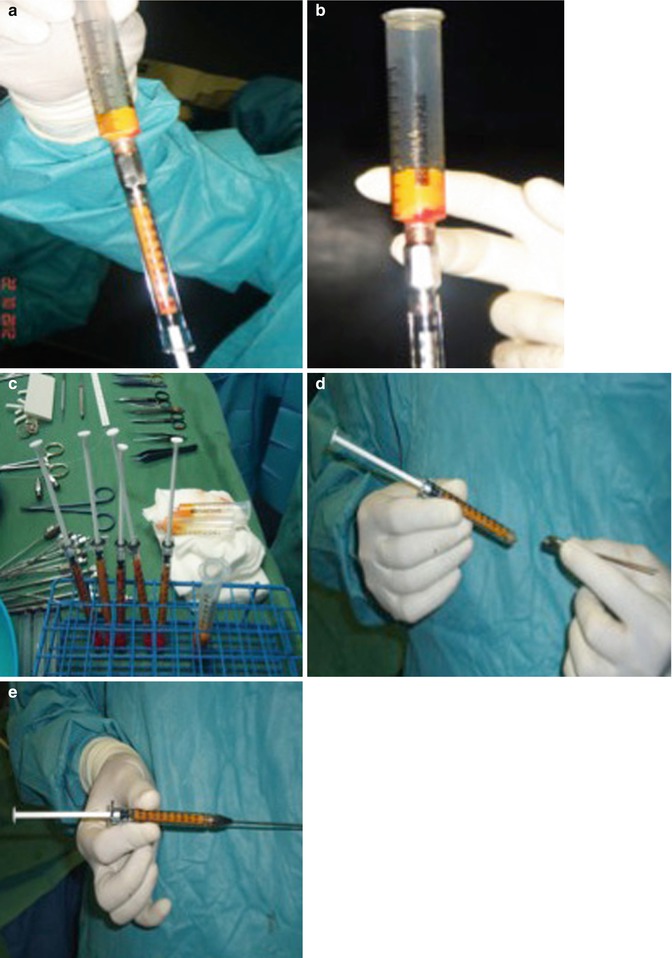

Fig. 30.7
(a–e) Transfer of the fat into Luer-Lock syringe
Placement of the refined fat into the recipient site is the most challenging part of fat grafting. Fat parcels should be positioned to ensure uniform survival, stability, and integration into the surrounding recipient tissue. The key of fatty tissue placement is maximization of the surface area of contact between the harvested fat and the recipient tissues. On the other hand, injection of a mass of fat into any site may result in areas of fat that are too far from vascularized tissue to have a source of nutrition or respiration. In this event, much of the tissue will die or resorb. This may result in irregularities. We advance the infiltration cannula through the tissue to the appropriate plane. Once the cannula is in the desired location, we press the plunger of the 1-mm syringe slightly while the cannula is being withdrawn. Then, the authors leave fatty tissue in the pathway of the retreating cannula, permitting more stable and regulated placement and minimizing the potential for irregularities or clumps of tissue.
Placement of minuscule amounts of fatty tissue with each pass is critical to successful structural fat grafting. To maximize the surface contact area, the largest amount of tissue that should be placed in the face with each withdrawal is 1/10 mL and much smaller aliquots (the maximum placed should be closer to 1/30 mL or even 1/50 mL per withdrawal) should be placed in specific areas, such as the periorbital region. Placing the fatty parcels with a blunt cannula that causes minimal disruption of the natural tissue planes facilitates better fat adherence to the recipient site. The surgical technique we used is a multilayered full facial augmentation using relatively high volumes of fat. Full facial rejuvenation is performed more often to maintain or establish normal facial proportions. When fat is used for regional augmentation, such as the midface, small volumes of fat are injected to this area compared with the volume that would have been used if they were a part of full facial augmentation. With full facial augmentation, we inject larger volumes to each region because adjacent areas are also treated.
There are key regions of the face that must be augmented with fat to create a more youthful appearance. Primary areas of treatment include the midface: infraorbital region (which is the major support area of the eyelid and face), anterior and lateral cheek, brow and upper eyelid, prejowl sulcus, and mandibular angle. Proper augmentation of the midface also blends into the lateral eyebrow, the anterior and inferior temple, and the submalar regions. Other key regions are the lateral jawline, the temporal fossa with the lateral eyebrow, the perioral region (lips, nasolabial fold, and prejowl), and the glabella.
The authors’ patients’ ages ranged between 18 and 88 years old in both females and males. Autologous fat grafting can be performed on a patient before facelift or blepharoplasty surgery and may prolong the need for such procedures.
The most successful areas of fat grafting are the more static ones such as the tear trough deformity, the midface, the upper malar region, the jawline, the temporal fossa, the mandible, and the lateral eyebrow. Fat grafts that are placed in the perioral region are viable, but the result is less predictable because of the mobility associated with the area. This results in shearing forces, which limit the angiogenesis to the graft. The best candidates for fat grafting are younger (<50 years old), healthy, nonsmokers, and with thicker skin and good overall skin elasticity. Smoking decreases the revascularization potential for the graft. Thinner, inelastic skin in older patients also does not contain optimal vascular supply and may require more than one treatment session for adequate results.
30.10 Influence of Local Anesthesia on Adipose Tissue
The most common modality the authors use for harvesting fat is local anesthesia with sedation. There have been concerns about the effect of local anesthetics such as lidocaine on the viability of fat grafts harvested by any kind of liposuction since such an agent is routinely used for analgesia of the donor site. Such a concern is based on an in vitro study reported by Moore et al. that lidocaine may inhibit a variety of adipocyte functions in tissue culture. However, the effect is found to be totally reversible once the agent has been washed out [52]. The viability and differentiation of preadipocytes are also found to be impaired but only after being isolated from fat tissue and then exposed directly to a higher concentration of lidocaine (2 %) in vitro for 30 min. The effect is thought to be independent of lipophilic properties and the resulted in vivo concentration of lidocaine may be different due to dilution effects [53]. However, Shoshani et al. [54




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








