Fig. 42.1
Preoperative
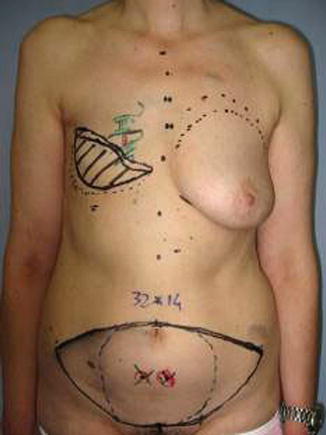
Fig. 42.2
Preoperative markings
Perforators are detected preoperatively by angio-CT and marked accurately in the skin island. Optionally these can be marked with the pencil Doppler.
42.2.2 Operative Technique
The patient is placed supine with both arms abducted. A two-team approach is used in every case. While the first team is raising the flap, the second team prepares the skin pocket on the chest wall and the recipient vessels in the thorax.
The lower abdominal wall is made first, and the superficial inferior epigastric vessel is identified in both sides and dissected for some centimeters. This vessel can be potentially used to improve the venous outflow in case of venous drainage insufficiency. The flap is usually elevated from lateral to medial, and dissection of the perforators is begun when the angio-CT-selected vessel is reached. The dissection is performed with bipolar forceps and micro scissors. Then the anterior rectus fascia is opened, and the rectus abdominis muscle is split longitudinally to perform the vessel dissection, while muscular branches are coagulated and every nerve is preserved (Fig. 42.3). The dissection proceeds on the lateral border of the rectus muscle where the inferior epigastric pedicle is dissected down to the external iliac vessel to obtain the desired length of the pedicle and comfortable vessel diameter (Figs. 42.4, 42.5 and 42.6).
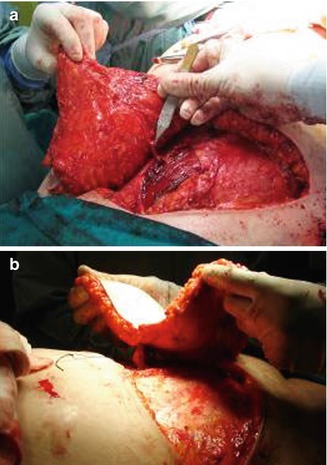
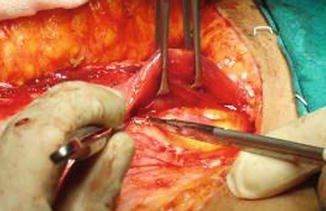
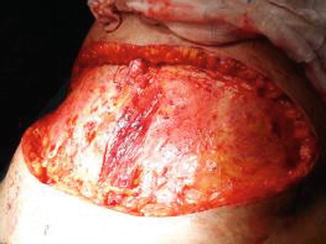
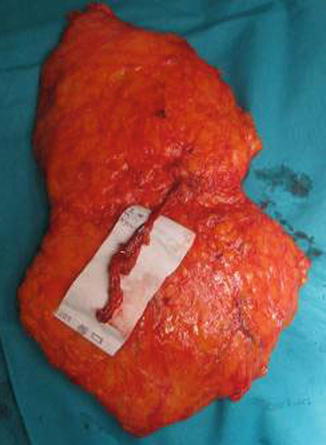

Fig. 42.3
(a, b) Pedicle dissection trough the muscle after opening the rectus fascia

Fig. 42.4
Pedicle dissection on the lateral border of the rectus

Fig. 42.5
The abdomen after flap removal

Fig. 42.6
The DIEP flap before the microanastomoses
The flap is transferred and sutured into the chest wall defect to fill the postmastectomy pocket, and the microanastomoses are performed with the internal thoracic vessels. The anterior rectus muscle and sheath is closed primarily, and the abdominoplasty and the umbilicoplasty are performed (Figs. 42.7, 42.8 and 42.9).
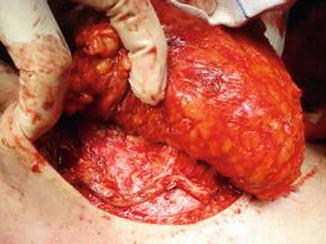
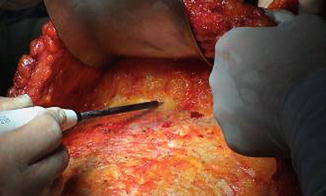
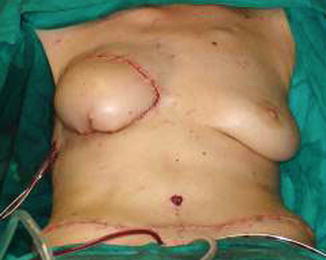

Fig. 42.7
Anastomoses performed with the internal thoracic vessel

Fig. 42.8
Abdomen undermining

Fig. 42.9
Final result after suturing
42.2.3 Fat Harvest and Injection
Marking of the areas for liposuction and fat grafting is done while the patient is standing. Fat harvesting is performed with the Coleman technique [15–19].
The donor sites (super-umbilical, flank, knee, and thigh) are infiltrated by a mixed solution (0.5 % lidocaine with 1:200,000 of epinephrine in lactated Ringer solution) (Fig. 42.10). The solutions are infiltrated at a ratio of 1 mL of solution per cubic centimeter of fat grafts to be harvested.
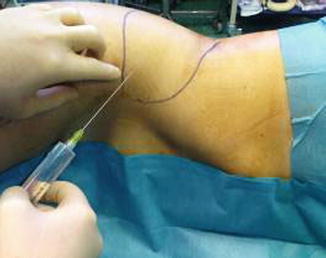

Fig. 42.10
Donor site infiltration
Fat is harvested from the donor areas by a cannula with a blunt tip inserted through a small incision in the selected area to be lipoaspirated (3 mm diameter; 12, 15, or 23 cm length; and two to three blunt openings) connected to a Luer-Lok syringe (20 mL) (Fig. 42.11). Incision is made by a number 11 blade and liposuction is performed (Figs. 42.12, 42.13 and 42.14). After filling the syringe with harvested tissue, the cannula is removed and the harvested tissue placed into a 10-mL syringe and centrifuged at 3,000 rpm for 1 min (Fig. 42.15). Now the sample is separated in an oily layer (upper level), a middle layer composed of the adipose tissue graft, and the aqueous layer (lower level) (Fig. 42.16).
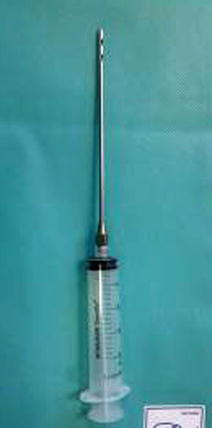
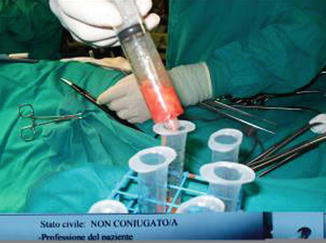

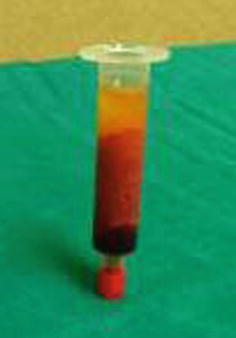

Fig. 42.11
Cannula connected with a Luer-Lok syringe

Fig. 42.12
Skin incision

Fig. 42.13
Fat harvesting

Fig. 42.14
Fat collection in 10-mL syringes

Fig. 42.15
Centrifugation of the harvested fat at 3,000 rpm for 1 min

Fig. 42.16
The three layers of the sample after centrifugation
The top and bottom layers, the oily and aqueous, respectively, are drained out of the syringe (Fig. 42.17).
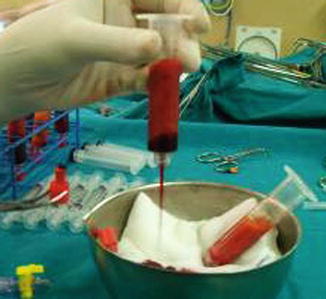

Fig. 42.17
Removal of the oily and aqueous layers
The fat graft is transferred in 3-mL syringes and injected in small amounts as the needle is gradually withdrawn from the reconstructed breast (1 mL injected every 1 cm2) (Figs. 42.18 and 42.19). The incisions in the flap are made using a 17-gauge needle that makes an adequate access without any visible scar. It is necessary to create micro tunnels in many directions in order to reach a well-vascularized recipient tissue and avoid areas of fatty pools that would lead to fat necrosis.
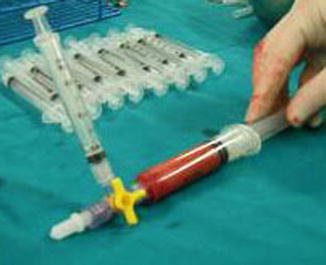

Fig. 42.18
Fat transferred in 3-mL syringes

Fig. 42.19
Fat syringes before the graft
Every fat injection was performed at least 6 months after the DIEP flap reconstruction with no particular attentions were made in order to avoid perforator vessels vascular supply. Fat injection is interrupted when the recipient site is saturated to avoid the risk of inducing areas of fat necrosis.
Resorption of about 30–40 % of the volume transferred can be expected.
In DIEP reconstruction, these are the main indications:
1.
Volume deficit









