20 Facial transplant
Synopsis
 Raising field with unanswered questions.
Raising field with unanswered questions.
 Necessity of lifelong treatment.
Necessity of lifelong treatment.
 Should be limited to patient with total destruction of orbicularis muscle.
Should be limited to patient with total destruction of orbicularis muscle.
 Indications are separated in two: Lower face transplant for destruction of orbicularis oris mostly indicated in ballistic traumas, full and upper face transplant indicated in destruction of orbicularis occuli mostly found in burned patients.
Indications are separated in two: Lower face transplant for destruction of orbicularis oris mostly indicated in ballistic traumas, full and upper face transplant indicated in destruction of orbicularis occuli mostly found in burned patients.
 Necessity of collaboration with many teams and strong logistic organization.
Necessity of collaboration with many teams and strong logistic organization.
Introduction
Face transplant is a new rising field in plastic surgery, with only 20 transplants from November 2005 to February 2012.1–9 However, six of those were realized in the year 2009, showing a great increase in their numbers. It is apparent that this will continue in the near future, but as in any innovative procedure, questions remain unanswered and only long-term follow-up will allow us to find those answers. Answers to the questions of risks–benefits balance; indications, and technical, immunological and ethical aspects are being progressively sought. Even if the number of face transplants is still small, the growing world expertise in composite tissue allotransplantation (CTA) is helping to answer some of these questions. CTA of various organs, such as the hands,10 face,1 bones,11 joints,12 abdominal wall,13 represents over 100 patients today, with some of them having over 10 years of follow-up. CTA transplants are related to nonvital organs, mostly to restore function. The goal of the face transplant is to restore human appearance, which should not be limited to a static aspect but should also be a dynamic one. This dynamic transplant allows the restoration of communication capacity and social reintegration.3
Basic science
Immunosuppression
We easily forget that the first kidney transplant in France only survived 3 weeks,14 as was the case for the first heart transplant15 and the first liver transplant.16 It is the understanding of Joseph Murray that the rejection was mostly due to an immunological phenomenon which led to intense research in immunology.17 In 1972, Joseph Murray18 explained his views on organ replacement and restoration of facial deformities, in his discourse to the Massachusetts Medical Society. Kidney transplantation was a developing field at that time, and face transplantation was not even a concept. Immunology was not then ready for CTA. In 1963, the first hand transplant was attempted and did not survive more than 3 weeks19,20 because of lack of immunosuppressive therapy at the time. In the years after the first heart transplant, the mortality rate was such a concern that many teams abandoned the principle, even though the surgical technique was well established. Starting in 1976, cyclosporine21 significantly improved the survival rate of patients by combining effective prevention of graft rejection and lack of hematologic toxicity. Other immunosuppressive agents introduced in recent years (tacrolimus,22 mycophenolate mofetil,23 monoclonal antibodies,24,25 antilymphocyte serum26), have expanded the range of available therapeutic drugs in organ transplantation, allowing to cope with almost all situations of rejection. The standard immunosuppressive treatment of CTA is a scheme suitable for high immunological risk transplantation such as lung transplants.27 It combines induction most of the time with antilymphocyte serum, which will cause lysis of effector cells responsible for acute cellular rejection, CD3+ T-lymphocytes, followed by maintenance therapy consisting of a three immunosuppressive drugs. Long after the first experimental work of Medawar,28,29 the skin appeared to be the ultimate barrier requiring anti-rejection drug too toxic to be tolerated long term. Furthermore, the complexity of the human immune system and the presence of the immunocompetent element in transplanted tissue during CTA30,31 could even be a threat by inducing graft-versus-host disease (GVHD) due to the presence of bone marrow and/or lymph nodes potentially involved in immune response. Since 1990, numerous studies were conducted in animals and in 1998, a team from Louisville showed that an immunosuppressive therapy combining Tacrolimus, Mycophenolate Mofetil and prednisone, could prevent the rejection of a complete transplanted limb, without major toxicity in a pre-clinical animal model (adult pig).32 All this knowledge has led to relatively standardized protocols. Thus, the Lyon team, led by Dubernard10,33 renewed the attempt to graft a hand, first unilateral then bilateral. If the unilateral graft eventually resulted in failure, this was due to the discontinuation of the patient, the first bilateral hand transplant was undoubtedly a success that has brought CTA to a clinical reality.34
We will focus here on the practical aspects of the treatment used in CTA. Today the standard treatment begins with an induction by injection of monoclonal or polyclonal antilymphocyte antibody. There is insufficient data so far between the choice of a monoclonal versus a polyclonal antilymphocyte antibody.35 In practice (Table 20.1), injection of antilymphocyte serum begins in the operating room just after the end anastomosis. The induction is then followed by a tri-therapy combining mycophenolate mofetil (MMF) and FK506 (tacrolimus) and steroids. The monitoring of this treatment requires accurate dosing in the days after surgery. Antilymphocyte serum needs a daily dosage of CD3+ T-lymphocytes. For tacrolimus, it is necessary to have a blood level of 10–15 ng per mL. For the Cellcept, repeated testing is performed to estimate the average concentration by using the area under curve (AUC) concept, which should be in the range of 40–50 ng/mL.
Other treatments are currently under study.36 Apart from drugs, research protocols are directed towards tolerance induction. The concept was mostly developed by Starzl.37–39 The principle is to prepare the donor or the graft so that the antigens of the transplanted organ are recognized as the antigens of the recipient. Many protocols exist in animals, but most involve a preparation by radiation which is not compatible with clinical practice. Recent approaches applicable in humans consist of a concomitant injection of hematopoietic stem cell from the donor. This bone marrow transplant can be a myeloablative protocol, i.e., removing the bone marrow recipient, which will change the immune system of the recipient and may cause a GVHD reaction. Thus, although particularly effective myeloablative approach is only used if there is a need for bone marrow associated to a solid organ transplant, as in the case in patients with myeloma with kidney failure, it is not applicable in the field of functional transplantation, as are CTAs. Nonmyeloablative protocols have been used in many animal models with success,40,41 but we do not have conclusive results in humans. For some, the presence of bone marrow in the bones of the donor’s hand is a true hematopoietic stem cell transplant and would explain the good results of these grafts. However, the comparison between the first three facial CTAs shows similar results in terms of immunosuppression with or without hematopoietic stem cells transplantation, as in the first transplant, or without, as in the two following. For others, the bone marrow transplant is only effective when transplanted with a vascularized bone which is the case in the hand and in transplants with mandible. This is not the case for the maxilla due to the relative paucity of bone marrow in the maxilla.
Some more subtle approaches seek a peripheral tolerance by treatment of blood cells trying to reduce the response of T-cells by causing antigen-presenting cells to a tolerogenic phenotype through apoptotic elements. This is the case of extracorporeal chemo-phototherapy (ECP),42–44 where leukocyte cells are irradiated with UVA after leukapheresis and pretreated with psoralen. The principle of ECP is to induce apoptosis with UVA radiation after their presentation by psoralens. These leucocytes are immediately reinfused into the patient, where they undergo early apoptosis. Following apoptosis, the leucocytes are engulfed by macrophage or other antigen presenting cells such as immature cells in an anti-inflammatory environment. The anti-inflammatory pattern and the engulfment by immature cells without co-stimulatory molecules induces anergy by deleting effector T-cells that respond to the presented antigens. An increase in regulatory T-cells is also induced after ECP and may contribute to the allograft acceptance by the recipient. ECP has already been used for the great majority of solid organ transplantations to cure acute rejection episodes or in an attempt to prevent or cure chronic rejections.
For Starzl,35–37 a pioneer of liver transplantation, tolerance should not be sought immediately, but should rather be a progressive approach through immunomodulation with rapid decline of anti-rejection treatment after the induction phase and adapted control of rejection. In light of his experience in transplantation of kidney and liver, he explained that in some cases, we may obtain a long-term safe treatment with low or no immunosuppression, by allowing the rejection to occur but by controlling it fast before the immune system is overwhelmed or the graft is damaged. The rejection will not only stimulate toxic T-cells but also regulatory cells. A long-term tissue chimerism should then occur even if never demonstrated in the transplanted patient. The results observed in our long-term follow-up in our team, are in favor of this hypothesis, as we have obtained a state of immunological stability, despite the strong decrease of immunosuppressive therapy.
Face transplant only necessitates blood type compatibility. However, HLA matching can be a necessity in the case of patients who are sensitized, as seen in the burn patient who has received cadaver allograft, or after blood transfusion.45 Each screened patient should have an anti-HLA serum test every 3 months during the preoperative period. HLA antibodies can appear also even in the absence of any recognized transplant, as certain viruses can mimic HLA antigens.46 This explains the occasional apparition of these antibodies when very sensitive detection methods as Luminex47 are used. In the case of a high level of positive HLA antibody, desensitization can be attempted but is often inefficient.48 A virtual preoperative cross-match should be made and this has to be negative to allow transplantation, followed by postoperative real cross-match. If real cross-match is positive, then specific treatment by anti-CD20 monoclonal antibody and injection of immunoglobulin should be made to avoid humeral rejection.
Surgical technique
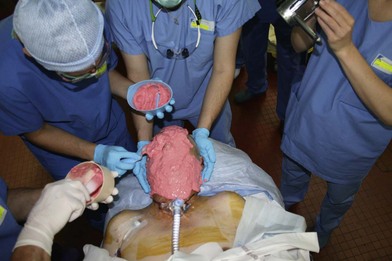
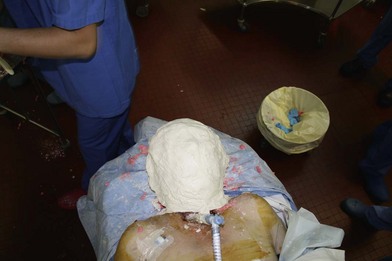
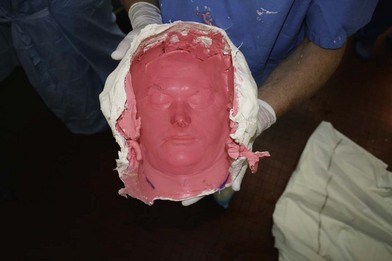
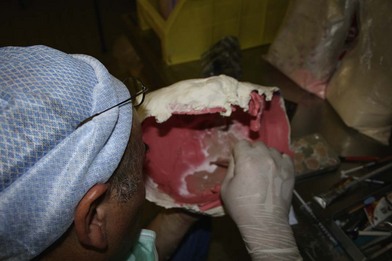
While each recipient had particularities due to individual defects, common aspects of all the transplant harvests can be described. In all of our cases, the facial transplant is the first tissue harvested from a heart-beating brain-dead donor. Tracheotomy followed by alginate molding of the donor’s face is done prior to any harvest, to allow reconstitution of the donor after harvesting. The harvest before cardiac arrest seems to be the most used technique. Contrarily to the hand or internal organ, the harvest of the face is a long procedure and the harvest on a nonbeating heart would lead to a very long warm ischemia. A long ischemia time could compromise the muscle function and could also change the immunologic impact due to the ischemia reperfusion injury, but improvement in organ preservation and surgical technique may prevent this in the future. Dissection on a beating heart donor helps to identify small vascular branches that could bleed if not correctly coagulated at the time of harvest. However, the restitution of the donor is fundamental and the molding should be done before any surgery. As the head and neck region is in the operative field; the anesthesiologists have then access to only the lower part of the donor. This disposition makes simultaneous removal of other organs impossible, even if all the transplant teams can, in theory, operate at the same time. The graft is washed with heparin-containing saline and transported in a standard icebox in preservation solution. Several organ preservation solutions have been used by different teams but none of them have been studied specifically for CTA. Once all the organs have been harvested and the resin mask, prepared during the operation, is fitted to the donor’s face, the cadaver is returned to the family. We have tested several methods of reconstitution and the use of hard resin is the most efficient, as it can be done in a similar time as the harvest (Figs 20.1–20.5).
Face transplant harvest
We recommend developing a reproducible and standardized technique.49,50 The best technique is to harvest a full face transplant and to remove the unnecessary elements for the recipient. In this way, as all the elements are brought from the donor, the recipient team are confident as to what to remove on the recipient. Moreover, the reconstitution of the donor is easier. Basically, the drawing (Fig. 20.6) goes from the scalp as a coronal incision; goes behind the ears and down to the neck, where a large skin flap has to be elevated and is only limited by the tracheotomy. The dissection starts in the neck with the approach of the external jugular vein on each side (Figs 20.7, 20.8). This vein has to be dissected as low as possible to allow its use on the recipient. It is divided to have access to the deep vessels. The carotid artery and vein are isolated and followed upward. The thyrolingofacial trunk is then isolated (Figs 20.9–20.11). To have access to the different vascular branches, the hypoglossal nerve and the posterior part of the digastric muscle has to be divided (Fig. 20.12). This has to be done on both sides before proceeding to the next step. The facial nerve is approached through a parotidectomy access route. It is dissected until its bifurcation and transected at its exit from the stylomastoid foramen (Fig. 20.13). A 8-0 nylon is used to facilitate its location once on the recipient. To facilitate the approach, the external auricular conduit is transected. This way the facial nerve can be seen well and the maximum of nerve can be harvested. At that stage, several branches of the external carotid are still holding it back. The pharyngeal and lingual arteries have to be divided. One should take great care not to cut the facial artery at that stage, as it is running deep in the neck. The posterior auricular artery should be raised with the flap to allow scalp blood supply. For face transplant including the upper part and the scalp, the incision is sagittal and the flap is raised from posterior to anterior, followed by the forehead and including both ears (Figs 20.14, 20.15). The sagittal incision allows the harvest of the entire scalp. It is not necessary to harvest the occipital arteries. The dissection of these arteries is difficult and rarely reproducible, as they run very deep behind the sternocleidomastoid muscle. Our anatomical studies revealed that the superficial temporalis pedicle can perfuse at least 80% of the scalp and that the posterior auricular arteries are more important than occipital artery for scalp blood supply. The sagittal incision runs from the occipital area to the vertex. The scalp is dissected on a subperiosteal plane from backwards to forwards, as in a coronal approach. If the scalp is not necessary, a standard coronal approach is done. At that stage, the flap holds laterally by both maxillary arteries and vein just at the temporomandibular joint level. These vessels are ligated on both sides, which helps to have access to the orbital region (Figs 20.16, 20.17). On the upper part of the flap, the dissection goes down to the levator muscle, which is divided after positioning a 3-0 nylon for easier repair (Figs 20.18–20.21). The flap is then raised towards the front, following the masseter plane (beneath its aponeurosis) to the oral mucosa, along with the facial nerve, the parotid gland – including Stensen’s duct, the superficial portion, and part of the deep portion – the mental nerve, the orbicularis oris, and most of the smile muscles (including both the zygomaticus and levators). At the anterior edge of the masseter muscle, the dissection plane is subperiosteal at the level of the horizontal branch of the mandible and on the malar bone and the zygoma. The mental nerve is identified at its exit from the mental foramen and communicates with the oral cavity at the level of the cheek. The entire cheek mucosa is harvested along the parotid ostium. The buccal fat pad is left behind. On the dental part of the maxilla and mandible, the mucosa is cut at the gingival. The lateral canthus on each side is also divided after insertion of a 2-0 steel stitch, which will allow reinsertion (Fig. 20.22)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree