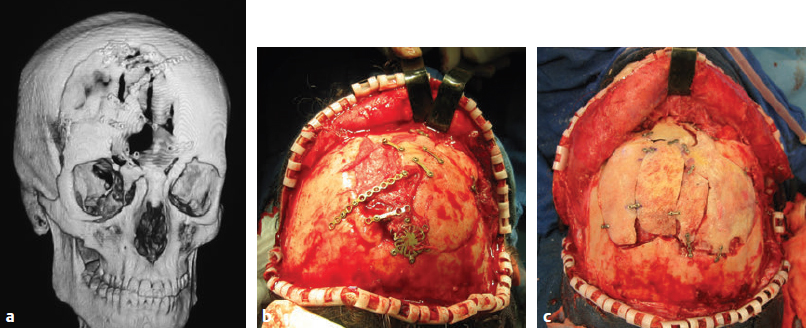
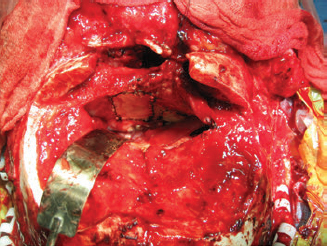
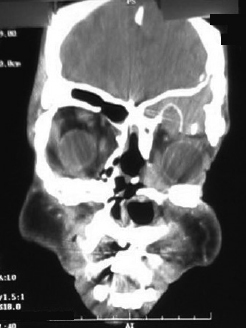
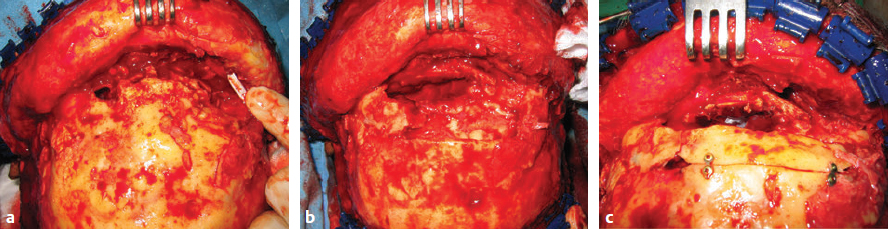
CHAPTER Traumatic injuries to the craniofacial complex can be devastating. Long-term sequelae lead to disfigurement and dysfunction if not managed appropriately. Trauma to the face can involve soft tissue, bone, salivary glands, or sensory organs. Initial management of facial trauma follows Advanced Trauma and Life Support guidelines. Once stabilized, facial injuries are treated. To optimize outcome, a systematic approach should be undertaken: 1. Accurate diagnosis by physical examination and supporting radiographic studies 2. Formulation of optimal surgical timing as well as methodology of surgical repair 3. Proper execution of treatment plan, including: • Adequate surgical exposure while following basic aesthetic principles • Anatomic reduction • Stable fixation • Optimal soft tissue management and resuspension 4. Early mobilization and proper rehabilitation Despite best efforts, treatment results of facial trauma may be suboptimal. Reduction may collapse over time because of cicatricial or muscular forces. Bony malunion or nonunion may occur. There may be poor scar formation or neurologic injury secondary to operative treatment. Unfavorable outcomes should be approached in a similar fashion to the initial injury. A methodical approach that begins with a physical examination supported by radiologic studies followed by goal-directed surgery leads to success. H.K. Kawamoto (personal communication, 2016) reiterates, “You have to know what the problem is before you can fix it.” This chapter discusses the treatment for unfavorable results of upper and midface fractures. Although rare in the facial area, infection is the first complication surgeons must watch for postoperatively. The time course may be in the initial days after the operation, but infection may not present until weeks or months later, in the form of hardware infection and exposure. If signs of infection are noted in the acute phase of healing, then simply releasing sutures or staples and gently reopening portions of the wound can be beneficial. This allows egress of infected material and exposes the wound to oxygen, inhibiting anaerobic bacterial growth. Many practitioners choose to treat perioperatively with a 5- to 7-day course of oral antibiotics that covers oral flora. For an infection that is more physiologically severe or when clinical evidence of an infection progressing beyond a localized wound is present, hospital admission is indicated. Treatment includes volume resuscitation, parenteral antibiotics, and débridement of infected tissues. Hardware infections present either in the early or late stage as exposure or purulence around plates and screws. Hardware exposure can often be treated conservatively until bony healing is complete. If bony union is not achieved, the hardware pockets are irrigated of frank purulence and débrided conservatively. The wounds can then be reclosed over a drain and a course of antibiotics given. After bony union is achieved, the hardware can then be removed. Osteomyelitis is uncommon in facial bones but can occur after excessive periosteal stripping and contamination of a fracture. Osteomyelitis presents with localized (rubor, calor, dolor) or systemic (fever, myalgias) infection. Osteomyelitis is diagnosed by a combination of bone biopsy and specific radiographic findings.1,2 Management of osteomyelitis includes surgical débridement of affected bone, removal of all internal hardware, maintaining bone orientation, and 6 weeks of intravenous antibiotics.3,4 The remaining bone segments’ orientation may be maintained by external fixation devices or replacement of plates. If the surgeon chooses to replace the internal plates, then load-bearing reconstruction plates are preferred with locking screws placed away from the site of infection in well-vascularized, healthy bone. Summary Box Unfavorable Results and Complications in the Treatment of Facial Trauma • General • Frontal sinus fractures • Naso-orbitoethmoid fractures • Orbital fractures • Zygomaticomaxillary complex fractures • Nasal bone fractures • Le Fort fractures • Panfacial fractures Scar formation from traumatic soft tissue injuries affects the result as much as bony reduction. Often, cicatricial formation of the face leads to aesthetic and functional impediments. Lacerations with crushed edges, avulsed hypoperfused superficial tissue, increased bacterial burden and local inflammation, and tight wound closure all combine to increase scar formation. To optimize cutaneous wound healing, patients should be advised to avoid sun exposure and perform scar massage or topical treatments. Massage mechanically softens the scar tissue and counteracts cicatricial thickening and contraction. Silicone gel or sheets improve scar contour and thickness.5,6 These interventions can be started after the removal of suture, provided the wound is healing well, typically after 10 to 14 days. Hypertrophic scarring appears at 3 to 4 weeks and can potentially be inhibited by serial intralesional steroid injection.7,8 This intervention will act locally to inhibit scar tissue formation. Immunomodulators such as 5-fluorouracil have also been shown in some studies to be effective.8–10 Laser and phototherapy may decrease the prominent redness of scars.11,12 If an unaesthetic scar persists beyond 6 to 8 weeks despite optimal scar therapy, scar revision and excision may be performed. This may be performed as a simple excision or other forms of local tissue rearrangement. The timing for revisions generally allows 3 to 6 months of healing to permit full resolution of tissue inflammation. As is the case with any elective procedure, it is important to medically optimize the patient for wound healing. The patient should be nutritionally optimized, tobacco free, and under adequate glycemic control. Full scar excision removes chronic inflammation and allows closure with noninflamed tissue. The wound should be designed in a way to hide it within the resting tension lines of the face, as well as to allow local tissue recruitment and tension-free closure. Abnormal fracture healing results in delayed union, nonunion, or malunion. Delayed union is slow or delayed bone healing. This occurs in cases with prolonged tissue inflammation, infection, or insufficient bone stabilization. Insufficient stabilization may be caused by screw loosening, fractures of the bone adjacent to hardware, or plate fracture. Delayed union becomes nonunion when bone fails to ossify after adequate healing time.13 A nonossified fibrous bridge often forms between bone ends and is mechanically insufficient to support the fractured segments, resulting in structural collapse against adjacent muscular or cicatricial forces. Nonunion is more likely to occur in the mandible than in the maxilla, where the forces supported are greater. Malunion is nonanatomic postreduction alignment of proximal and distal bone.14 Malunion follows poor reduction as well as hardware loosening and failure. Malunion presents with clinical findings of a step deformity at fracture segments, malocclusion, or an asymmetrical facial contour. The evaluation of malunion or delayed nonunion starts with a physical examination. It is appreciated by facial bone mobility, step-off, and asymmetry. The physical examination is followed by radiographic imaging. Computed tomography (CT) provides a thorough evaluation for bony gaps and nonviable bone. If vascularized bone flaps are necessary, CT angiography can confirm donor vascular anatomy of the flap. In these cases, the patient should be fully evaluated for physiologic tolerance for prolonged surgery. The treatment for bony nonunion involves removing the previously placed hardware and excising the fibrous bridge. Bone, as free or vascularized graft, is then used to bridge the segment, and the entire construct is supported by hardware. The length of the bone gap is important because it determines the treatment. Nonvascularized or allogenic cadaveric bone grafts adequately reconstruct defects up to 6 cm, whereas vascularized bone grafts, from the fibula or iliac crest, are ideal for defects larger than 6 cm.15 For a defect smaller than 6 cm in a nonirradiated patient, treatment options include cadaveric or nonvascularized autogenous bone graft. Adding a substance such as bone morphogenetic protein (BMP) to this site may improve osteoinductive potential, through direct stimulation of bone growth and induction of bone stem cells. These nonvascularized corticocancellous grafts are secured to the bone plate within this continuity defect. Bony defects larger than 6 cm require reconstruction with vascularized bone flaps. With these large bone gaps, vascularized bone provides osteogenic potential for healing and prevents the resorption seen in conventional bone grafts. Possible donor sites include the fibula, iliac crest, scapula, and radius, each with their own set of advantages and disadvantages. Flaps may be transferred as bone and soft tissue composite free tissue to correct soft tissue deficits. The frontal sinus begins to develop at age 6 years. In adults, the sinus is completely formed as air-filled spaces in the forehead on either side of the midline. With an average total volume of 14 mL, the frontal sinuses are composed of an anterior and posterior wall and can exist as two separate spaces separated by a bony midline septum or a large communicating single cavity.16 Mucosa lines the bony cavity and produces secretions that drain through nasofrontal ducts traversing the inferomedial sinus floor and to the nasal middle meatus. The anterior wall forms brow and forehead contours, whereas the posterior wall forms the limit of the anterior cranial fossa. Fractures of the frontal sinuses have many patterns and can include one or both sinuses; involve the anterior wall, posterior wall, or both walls; and may or may not extend to or cause obstruction of the frontal duct system. Initial treatment involves three considerations that determine the outcome. The anterior wall supports the aesthetic contours of the brow and forehead. The posterior wall supports the anterior cranial fossa and dura. Fractures injure the dura and form a communication between the brain and nasal cavity. The nasofrontal duct drains the sinus, and obstruction can lead to trapping of secretions and infection. Management of these fractures focus on restoring integrity of the anterior walls but also on the creation of what has been conceptually referred to as a safe sinus.17,18 This concept refers to preventing mucous trapping by removing mucosal elements of the sinus when the nasofrontal ducts are compromised or intracerebral infection is of concern. The initial treatment of frontal sinus fractures starts with prophylactic antibiotics. Antibiotic choice should cover gram-negative, gram-positive, and anaerobic bacteria, such as clindamycin. Anterior table fractures are reduced and plated into anatomic position. Injury to the nasofrontal duct is treated by sinus obliteration, removal of all mucosal elements, and closure of the nasofrontal duct. Displaced fracture of the posterior wall is treated by (1) cranialization, (2) removal of the posterior wall, (3) removal of all mucosal elements, and (4) repair of dural injuries. Reconstructive failure of these four elements may create undesirable results. Contour irregularity of the forehead and glabella result from loss of soft tissue, bone fragment resorption, or inaccurate realignment of the bones. If this occurs, it may be noticeable and become a stigmata of previous injury, sometimes causing patient distress. Evaluation starts with physical examination followed by CT. Scans can determine whether the aesthetic abnormality is related to soft tissue atrophy or underlying bony irregularity. Bony irregularity in the form of overlapping step deformity or irregular thickness can be burred or filed down. Options to improve soft tissue contour defects include soft tissue grafts like fascia, dermis, or fat. For large or bony defects, bone grafts as well as alloplastic materials such as prefabricated polyethylethylene or porous polyethylene may be used. Each of the aforementioned materials has its proponents, depending on the size and type of contour. Autogenous options are superior because of a decreased potential for infection or granulomatous reactions. Overcorrection is indicated to account for the expected fat and bone graft resorption over the first year and beyond (Fig. 48.1). Fig. 48.1 (a,b) A young patient presented after posttraumatic frontal sinus and bone repair with significant frontal and cranial bone loss. (c) Split calvarial bone was used to reconstruct the frontal bone, glabella, and forehead. (These images are provided courtesy of J.I. Garri.) Meningeal tears and injury can result in persistent cerebrospinal fluid (CSF) leak and secondary CSF space infection. This can either result from poor healing and sealing of the dural closure or unidentified dural tears. Evaluation for a chronic CSF leak is a clinical diagnosis but can be verified by beta-transferrin levels of the fluid. The clinical presentation is persistent clear nasal drainage. CT may be useful to identify the location of the dural defect. Dural defects are repaired by direct suture or using dural patches of free fascial graft or alloplastic collagen sheets.19,20 A preferred method is the use of vascularized pericranium or temporalis to cover suture lines (Fig. 48.2). When there is significant dead space and continued CSF leak, pericranium and temporalis flaps may be insufficient. These situations may be best dealt with using free tissue transfer, which can fill dead space, improve vascularity to the region, and stimulate sealing of the dura.21 Mucoceles of the frontal sinus result from sequestered secretions caused by partial or complete nasofrontal duct obstruction. They occur when there is an unrecognized nasofrontal duct obstruction or because of retained sinus mucosa after obliteration or cranialization and may occur years after the initial injury.22,23 Infected mucoceles are called mucopyoceles. Mucoceles expand from continued mucous secretion and compress and distort surrounding structures. Diagnosis is clinical, based on a history of frontal sinus fracture, fevers, visual symptoms, and headaches. CT defines the patient’s anatomy and structural distortion caused by the mucocele (Fig. 48.3). Early treatment of mucoceles involves ample drainage, which can be performed endoscopically if identified before significant anatomic distortion. If endoscopic drainage fails or is not technically feasible, a craniotomy is required to remove all retained sinus mucosa. Through a coronal incision, a craniotomy allows access to the mucocele. The brain is separated from the mucocele, and any inflammatory tissues are removed. The posterior table segments are removed and any remaining sinus surfaces are burred to remove all mucosal rests. Distorted facial and cranial structures are reconstructed with autologous bone graft (Fig. 48.4). The dead space is then obliterated by a pericranial or temporalis flap.23 If these are too scarred for use or have been used already, or the dead space is large, the surgeon must consider alternate options such as free tissue to close off those spaces. Fig. 48.2 A collagen alloplastic patch is used to seal the dura. (This image is provided courtesy of J.I. Garri.) Fig. 48.3 After a frontal sinus fracture, this patient presented with a left-sided mucopyocele that destroyed the orbital roof and rim. (This image is provided courtesy of J.I. Garri.) Fig. 48.4 (a) After a frontal sinus fracture with mucopyocele formation, this patient’s anterior table had irregularity and openings into the sinus. (b) Resection of necrotic bone exposed the sinus and allowed débridement of the mucopyocele and separation from the brain. (c) Reconstruction is with split calvarial bone graft. (These images are provided courtesy of J.I. Garri.) The naso-orbitoethmoid (NOE) region is a complex focus of bony articulations involving multiple facial buttresses. Here the nasal bones and cartilages, maxillary frontal processes, lacrimal bones, orbital plate, ethmoid air cells, cribriform plate, and frontal bone adjoin. NOE fractures affect the shape and position of the nasal pyramid, root of nose, and medial canthus. Accurate reduction prevents contour and symmetry irregularity of the nasal sidewall. The grade of injury determines the surgical maneuvers necessary to correct the medial canthal deformities. This includes reduction or fixation of fractured segments and transnasal wiring. Telecanthus is an increased distance between the medial canthi.24 It is a soft tissue relationship and differs from hypertelorism, which is increased interpupillary distance.25 The normal intercanthal distance equals the length of the patient’s palpebral fissure, approximately 31 to 33 mm.26 This is a gender-specific and ethnically variable measurement. Malpositioned medial canthi is a challenging problem to correct. Poor medial canthal position and appearance can be caused by soft tissue secondary healing and bone malposition. The evaluation should include a keen physical examination correlated with CT to better define the bone and soft tissue relationship. Scarring and fibrosis of the soft tissue around the medial canthal tendon distorts its position, leading to degrees of telecanthus. Alternatively, the previous medial canthal wiring may erode the bone and allow the canthus to slip laterally. This situation is most easily addressed with tissue rearrangement using small, local flaps. Horizontal discrepancies can be corrected with V-Y advancement, whereas vertical discrepancies are addressed with z-plasty–style transposition flaps. These flaps should be full-thickness tissue down to bone. The flap controlling the canthal angle is then reanchored to bone by a transnasal suture or wire to maintain the new soft tissue position. The wiring traditionally is performed by coronal incision, but a transcaruncular approach is a less invasive. The wire is passed posterior and superior with slight overcorrection. Alternatively, if bone erosion is a concern, a small plate may serve as the fixation point for the transnasal wire. If the bones are malpositioned, then a bone-centric approach is required. This starts by removing previous hardware and re-creating the NOE fracture via osteotomy and correctly reducing the segments. If the medial canthal tendon (MCT) is not fixed to these segments, it may still require transnasal canthopexy to achieve the anatomic position. Of note, the technique is very difficult, and the best secondary treatment of telecanthus is not defined. Skin thickness and tissue draping of the canthal–sidewall junction can be worsened by edema and is a stigmata of the injury. Transnasally fixed bolster dressings on both canthal–sidewall junctions help control the edema and maintain the soft tissue. These should be left in place for 7 to 10 days. Lacrimal apparatus damage occurs either from the injury or during the initial repair. The normal course of tears flows from the upper and lower puncta, through the superior and inferior canaliculi traversing the orbicularis, into the lacrimal sac in the lacrimal fossa, and through the lacrimal duct, exiting under the inferior nasal turbinate. Considering that 90% of tear volume evaporates, injuries may never be identified. Approximately 17% of nasolacrimal duct injuries associated with NOE fractures require surgical correction.27 Evaluation starts with determining a history of excessive tearing postoperatively. Traumatic telecanthus may be part of the history, because it is associated with lacrimal duct obstruction.28 Postoperative eyelid malposition should also be considered, because eyelid incisions are commonly used in facial open reduction and internal fixation. Epiphora may be expected in the initial postoperative period from edema of the lacrimal apparatus, but persistence beyond the normal healing time of 6 weeks necessitates testing. CT imaging can help identify bony fractures involving or occluding the lacrimal duct. Jones (primary dye test) test confirms lacrimal duct obstruction, with a sensitivity of 97% and specificity near 100%.29 Jones test 1 is performed, in which fluorescein is instilled at the medial canthus and subsequently drained at the inferior turbinate onto a cotton applicator. A positive test shows the duct is patent. If the test is negative, Jones test 2 is performed, which involves cannulation of the lacrimal duct and injection of fluorescein under pressure. A positive test means partial obstruction or stenosis of the lacrimal apparatus exists. Dacryocystorhinostomy is the operative repair of nasolacrimal dysfunction. This procedure is performed classically through an external open technique or endoscopic transnasal approach. External dacryocystorhinostomy begins with a well-placed incision within the medial orbital soft tissue, medial to the MCT. Dissection is carried down to the periosteum over the lacrimal crest. Periosteum is cleared from bone anterior to the lacrimal fossa, and the lacrimal sac is identified by passing a probe through the puncta. An osteotomy is made anterior to the lacrimal fossa to expose the nasal mucosa with a high-speed bur. The nasal mucosa is then elevated from bone and incised. A mirrored incision is made into the lacrimal sac. Crawford tubes are passed through superior and inferior canaliculi into the lacrimal sac. The terminal ends of the Crawford tubes are then placed through the osteotomy into the nasal cavity. The tubes are trimmed to stay within the nasal vestibule and secured with suture. An ostomy between nasal and lacrimal sac mucosa is then created by suturing the open mucosal edges. The skin incision is closed in layers, emphasizing aesthetics. Crawford tubes remain in place for at least 3 weeks to up to 6 months to maintain patency of the surgically created nasolacrimal fistula. A significant posttraumatic deformity caused by NOE fractures is the loss of nasal projection. The contours of the nose are supported by the nasal process of the frontal bone, the nasal bones, the frontal process of the maxilla, the septum, and upper and lower lateral cartilages. Significant trauma to the NOE complex may result in depression of its subunits and loss of support for the nasal pyramid. This may lead to upper and middle nasal vault deficiency, concavity of the nasal dorsum, and loss of nasal length or tip projection. Further secondary nasal reconstruction is discussed in the Nasal Bone Fractures section. With facial trauma, the bony support may be significantly distorted, such as in NOE fractures, and overlying soft tissue may heal in an unaesthetic manner. The surgeon should be careful when closing the soft tissue envelope at the initial repair, because wound dehiscence and widened scars may complicate healing. The medial orbit and canthus is an area of concern when underlying skeletal contours are altered and soft tissue swelling results. Prolonged tissue edema causes tissue thickening and loss of fine soft tissue anatomy. This may result in epicanthal folds, blunting of the medial canthal angle, or disruption of the canthal tendon repair. This is best treated with prevention. A rigid bolster dressing is placed on both nasal sidewalls and secured transnasally with suture. A rigid dressing can be assembled using a halved aluminum foam nasal splint or rolled petroleum gauze. Secondary revision can be quite complex and rarely will restore premorbid aesthetics. The soft tissue is rearranged as outlined in the Telecanthus section. Forces transmitted hydrostatically through the globe or through adjacent bones fracture the orbital walls.30 They are also associated with high-level midface fractures like Le Fort II and III patterns. Although blow-in fractures occur, blowout fractures are more common and result in increased orbital volume, restricted extraocular movement, and visual disturbances. The globe is evaluated for injury before repair to prevent exacerbation of injury and blindness. At primary repair the goals of treatment are restoration of correct orbital volume and reduction of orbital rim step-offs. This is performed through transconjunctival, subciliary, and subtarsal incisions. The orbital wall defects are closed with either autogenous bone graft, resorbable and nonresorbable synthetic plates, or titanium plates. Orbital floor reconstruction plates are positioned on the posterior ledge of the fracture to accurately re-create volume. Poor outcomes from orbital fractures include ocular injury, diplopia, enophthalmos, eyelid malposition, and hardware complications. Ocular injury after orbital fractures is common, occurring in approximately 60% of cases.31 The incidence increases as the number of other associated facial fractures increases. Associated ocular injuries include hyphema, retinal injury and hemorrhage, ocular hypertension, optic neuropathy, and globe rupture.31 Delayed repair of the orbital fractures is necessary to allow these injuries to stabilize. Any of these preexisting injuries can progress to blindness if not managed appropriately and with caution. Ocular injury may also occur during the dissection of the orbital defects by blunt force. The optic nerve enters the superomedial orbit approximately 40 mm from the orbital rim. Iatrogenic complications are rare and are best treated with prevention. A rare complication after injury is sympathetic ophthalmia. This condition typically follows globe rupture. The clinical presentation results from a coordinated feedback response loop between injured excitatory and the uninjured sympathizing eyes. The injured eye presents with decreased vision stimulating photophobia, visual accommodation paresis, and pain in the sympathizing eye. The mainstay of treatment is enucleation within 2 weeks of injury upon failed improvement of vision. Medical treatments include high-dose steroids, immune modulators such as cyclosporine and mycophenolate, or intraocular injection of steroids.32 Diplopia results from the initial trauma or after surgical intervention. It is important to document diplopia and how it presents at rest or extremes of gaze. After orbital surgery, continued diplopia occurs in 8 to 30% of patients.33 Diplopia incidence increases in older patients after delayed orbital fracture repair.34 Immediate postoperative swelling of extraocular muscles may contribute to diplopia; following an interval for edema resolution, continued diplopia requires further workup. A thorough history and physical examination is performed, with ophthalmologic consultation, to diagnose the cause. Diplopia immediately after the initial reconstruction may be caused by plate impingement on extraocular muscles. Causes of persistent postoperative diplopia include intraocular pathology, extraocular muscle dysfunction, and discrepancies in globe position from enophthalmos or hypoglobus. CT will further define the extraocular anatomy and areas needing orbital volume correction. The treatment for diplopia depends on the cause, and subsequent correction of such typically ameliorates the visual disturbance. If diplopia persists, corrective lenses may be used to further improve symptoms. Although both conditions can occur concomitantly, enophthalmos is underprojection of the globe in the anteroposterior plane, whereas hypoglobus is caudad globe displacement into the space of the maxillary sinus. Both enophthalmos and hypoglobus cause extraocular muscle dysfunction by changing the axis of muscular action. Enophthalmos is quantified by two methods. A Hertel exophthalmometer directly measures the corneal lateral canthal distance. Immediately postinjury or postoperation, this measurement will be normal or exophthalmic because of periorbital swelling. Enophthalmos may not develop until several weeks after the injury. CT may be used to calculate orbital volume and approximate the difference from normal.35 Both of these conditions may cause periorbital asymmetry, cosmetic concerns, and disruption of the visual axis and can oftentimes be associated with diplopia. Postoperative enophthalmos and hypoglobus result from improper reconstruction of orbital volume, atrophy of traumatized orbital fat, or resorption of autogenous bone graft materials used in the repair. Orbital volume requires correction to resolve globe position problems. Causes include plate malposition projecting into the maxillary sinus, or poor plate adaption creating an oversized orbit. This reconstructive error must be revised by adapting the plates to correctly rest on the posterior ledge of the fracture, thus reducing orbital volume. The orbital volume can be fine-tuned with bone graft in addition to plates (Fig. 48.5).
48
Facial Fractures
Managing Unfavorable Results and Complications in Facial Fractures
Infection
 Infection
Infection
 Soft tissue injuries
Soft tissue injuries
 Malunion or nonunion
Malunion or nonunion
 Contour irregularity
Contour irregularity
 Meningeal tears and injury
Meningeal tears and injury
 Mucocele/mucopyocele
Mucocele/mucopyocele
 Telecanthus
Telecanthus
 Epiphora or disruption of the lacrimal system
Epiphora or disruption of the lacrimal system
 Cerebrospinal fluid leakage
Cerebrospinal fluid leakage
 Loss of nasal projection
Loss of nasal projection
 Epicanthal folds and soft tissue secondary abnormality
Epicanthal folds and soft tissue secondary abnormality
 Ocular injury
Ocular injury
 Diplopia
Diplopia
 Enophthalmos/hypoglobus
Enophthalmos/hypoglobus
 Complications related to the use of alloplastic materials
Complications related to the use of alloplastic materials
 Ectropion and lagophthalmos
Ectropion and lagophthalmos
 Malar flattening
Malar flattening
 Enophthalmos and lateral canthal dystopia
Enophthalmos and lateral canthal dystopia
 Sensory nerve damage
Sensory nerve damage
 Masticatory dysfunction
Masticatory dysfunction
 Airway obstruction
Airway obstruction
 Aesthetic deformity
Aesthetic deformity
 Septal complications
Septal complications
 Malocclusion
Malocclusion
 Nasal septal deviation
Nasal septal deviation
 Maxillary sinusitis
Maxillary sinusitis
 Cerebrospinal fluid leakage
Cerebrospinal fluid leakage
 Nerve damage
Nerve damage
 Loss of facial projection
Loss of facial projection
Soft Tissue Injuries
Malunion or Nonunion
Frontal Sinus Fractures
Contour Irregularity
Meningeal Tears and Injury
Mucocele and Mucopyocele
Naso-orbitoethmoid Fracture
Treatment Principles
Telecanthus
Epiphora or Disruption of the Lacrimal System
Loss of Nasal Projection
Epicanthal Folds and Soft Tissue Secondary Abnormality
Orbital Fractures
Treatment Principles
Ocular Injury
Diplopia
Enophthalmos and Hypoglobus
Plastic Surgery Key
Fastest Plastic Surgery & Dermatology Insight Engine