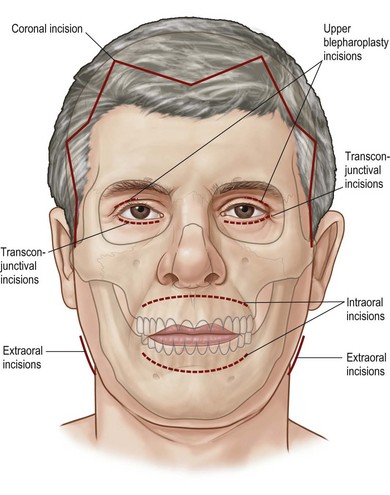
3 Facial fractures
Synopsis
 The teachings of John Converse, Nicholas Georgiade and Reed Dingman provided the benchmark for an entire generation of surgeons in facial injury repair.
The teachings of John Converse, Nicholas Georgiade and Reed Dingman provided the benchmark for an entire generation of surgeons in facial injury repair.
 The treatment concepts discussed in this chapter were developed at the University of Maryland Shock Trauma Unit and ultimately employed at the International Center for Facial Injury Reconstruction at Johns Hopkins.
The treatment concepts discussed in this chapter were developed at the University of Maryland Shock Trauma Unit and ultimately employed at the International Center for Facial Injury Reconstruction at Johns Hopkins.
 The proportion of severe injuries seen at these centers is high.
The proportion of severe injuries seen at these centers is high.
 The treatment concepts, however, may be modified for common fractures and less significant injuries.
The treatment concepts, however, may be modified for common fractures and less significant injuries.
 Greater emphasis has been placed on minimizing operative techniques and limited exposures, whereas the decade of the eighties witnessed craniofacial principles of broad exposure and fixation at all buttresses for a particular fracture across all degrees of severity.
Greater emphasis has been placed on minimizing operative techniques and limited exposures, whereas the decade of the eighties witnessed craniofacial principles of broad exposure and fixation at all buttresses for a particular fracture across all degrees of severity.
 Presently, the treatment of injuries is organized both by severity and anatomic area to permit the smallest exposure possible to achieve a good result (CT based facial fracture treatment).
Presently, the treatment of injuries is organized both by severity and anatomic area to permit the smallest exposure possible to achieve a good result (CT based facial fracture treatment).
Introduction
Over 4 million people are injured in automobile accidents in the United States yearly.1 Statistics on the number of facial injuries vary widely based on social, economic and geographic differences. The causes of facial injuries in the United States include motor vehicle accidents, assaults, altercations, bicycle and motorcycle accidents, home and industrial accidents, domestic violence and athletic injuries. The automobile is frequently responsible for some of the most devastating facial injuries, and injuries to the head, face and cervical spine occur in over 50% of all victims. Seatbelts and airbags have reduced the severity and incidence of facial injury, but primary and secondary enforcement of the laws vary in effectiveness with ethnicity, education and geographic location.2
History
In the 1980s, the application of craniofacial exposures improved the ability to restore the pre-injury facial appearance by providing access to the entire facial skeleton (Fig. 3.1). These techniques had their adverse sequela of soft tissue and nerve damage and displacement of soft tissue position on the facial skeleton. Current facial injury treatment minimizes potentially morbid exposures. The techniques of extended open reduction, immediate bone grafts, and microvascular tissue transfer have made impossible injuries manageable. The principle of immediate skeletal stabilization in anatomic position has been enhanced by the use of rigid fixation. Soft tissue position and volume over this expanded skeleton has been maintained, particularly preventing soft tissue shrinkage, displacement and contracture. These techniques improve the functional and aesthetic results of facial fracture treatment. It is axiomatic, however, that soft tissue incisions first be repaired in layers and then replaced at their proper position onto the facial skeleton.
In the last 30 years, the improvements in automobile construction and the traffic regulation have offered much protection from facial injury. The use of restraints, airbags and padded surfaces, the multi-laminated windshield, the improved design of rearview mirrors and steering wheels have all reduced the frequency and severity of facial injuries.1 Over the years, the popularity of the motorcycle still remains a factor in the etiology of major facial trauma. At the University of Maryland Shock Trauma Unit, the number of ballistic injuries has increased in proportion to the increase in drug trafficking. The character of ballistic injuries has also changed over the years from more destructive weapons to smaller caliber weapons.
Initial assessment
Timing of treatment
Timing is important in optimizing the management of facial injuries. Bone and soft tissue injuries in the facial area should be managed as soon as the patient’s general condition permits. Time and time again it has been the authors’ impression that early, skillful facial injury management decreases permanent facial disfigurement and limits serious functional disturbances.3,4 This does not mean that one can be cavalier about deciding who might tolerate early operative intervention. Indeed, the skillful facial surgeon must have as complete a knowledge of their ancillary injuries as well as those of the face. Classically, facial soft tissue and bone injuries are not acute surgical emergencies, but both the ease of obtaining a good result and the quality of the result are better with early or immediate management. Less soft tissue stripping is required, bones are often easily replaced into their anatomic position and easier fracture repairs are performed. There are few patients, however, whose injuries cannot be definitively managed within a short time. Exceptions to acute treatment include patients with ongoing or significant blood loss (i.e., pelvic fractures), elevated intracranial pressures, coagulation problems, and abnormal pulmonary ventilation pressures. Under local anesthesia, however, lacerations are debrided and closed, IMF applied and grossly displaced fractures reduced. Many patients with mild brain injuries or multi-system traumas do not have criteria preventing operative management. These patients may receive facial injury management at the time that other injuries are being stabilized. Indeed it is not uncommon in the University of Maryland Shock Trauma Unit for several teams to operate on a patient at the same time in several anatomic areas.
Clinical examination of the face
Blunt trauma craniofacial injuries
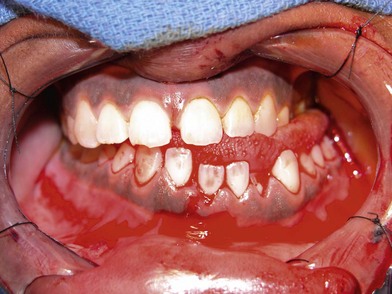
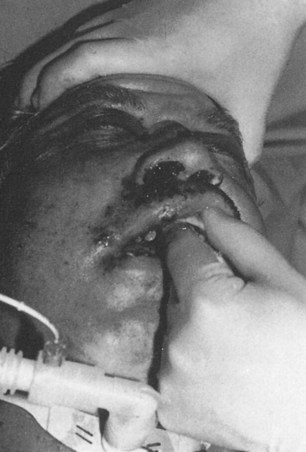
Symptoms and signs produced by facial injuries include: pain or localized tenderness; crepitation of bone movement; hypesthesia or anesthesia in the distribution of a sensory nerve; paralysis in the distribution of a motor nerve; malocclusion; visual acuity disturbance; diplopia; facial asymmetry; facial deformity; obstructed respiration; lacerations; bleeding and contusions. The clinical examination should begin with the evaluation for symmetry and deformity, inspecting the face comparing one side with the other. Palpation of all bony surfaces follows in an orderly manner. The forehead, orbital rims, nose, brows; zygomatic arches; malar eminence; and border of the mandible should be evaluated (Fig. 3.2). A thorough inspection of the intra-oral area should be made to detect lacerations, loose teeth or abnormalities of the dentition (Fig. 3.3). Palpation of the dental arches follows the inspection, noting mobility of dental-alveolar arch segments. The maxillary and mandibular dental arches are carefully visualized and palpated to detect an irregularity of the bone, loose teeth, intra-oral lacerations, bruising, hematoma, swelling, movement, tenderness or crepitus. An evaluation of sensory and motor nerve function in the facial area is performed. The presence of hypesthesia or anesthesia in the distribution of the supraorbital, infraorbital or mental nerves suggests a fracture along the bony path of these sensory nerves (cranial nerve V). Cutaneous branches of these nerves might have been interrupted by a facial laceration as well.
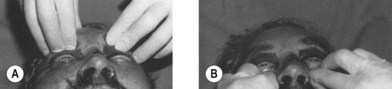
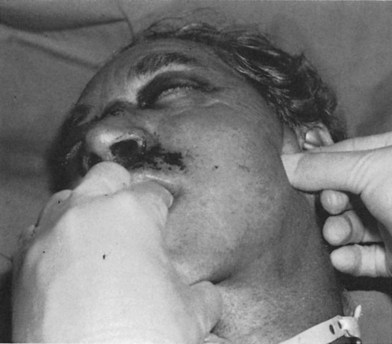
Extraocular movements (cranial nerves III, IV and VI) and the muscles of facial expression (cranial nerve VII) are examined in the conscious, cooperative patient. Pupillary size and symmetry, speed of pupillary reaction, globe turgor, globe excursion, eyelid excursion, double vision and visual acuity and visual loss are noted. A funduscopic examination and measurements of globe pressure should be performed. The presence of a hyphema, corneal abrasion, visual field defect, visual loss, diplopia, decreased vision, or absent vision should be noted and appropriate consultation requested. A penetrating ocular injury or globe rupture should be suspected where any laceration in the eyelids or periorbital area is present. The presence of a periorbital hematoma with the eye swollen shut should not deter a clinician from examining the globe. It should be emphasized, however, that gentleness must be exercised to avoid extrusion of lens or vitreal contents through a globe laceration by vigorous manipulation. It is only by means of a thorough clinical examination that globe ruptures and penetrating globe injuries are not missed. The excursion and deviation of the jaws with motion, the presence of pain upon opening the jaw, the relationship of the teeth, the ability of the patient to bring the teeth into occlusion, the symmetry of the dental arches and the proper intercuspal dental relationship are important clues to the diagnosis of fractures involving the dentition. One finger in the ear canal and another over the condylar head can detect condylar movement, or crepitus either by patient movement or when the jaw is pulled forward (Fig. 3.4). The presence of a gingival laceration or a fractured or missing tooth or a split alveolus should imply the possibility of more significant maxillary or mandibular injuries, which must be confirmed by CT. Fractures of the mandible may be detected by pulling the jaw forward or by applying manual pressure on the anterior portion of the mandible while supporting the angle. Instability, crepitus and pain may be noted when this maneuver is performed. Edema and hemorrhage may mask the perception of facial asymmetry. Bleeding from laceration of vessels accompanying facial fractures may disguise a cerebrospinal fluid leak. Bleeding or fluid draining from the ear canal may indicate a laceration in the ear canal, a condylar dislocation, or a middle cranial fossa fracture with a CSF leak. Bleeding from the nose may indicate nasal or septal injuries, Le Fort, nasoethmoidal, or orbital fractures or anterior cranial fossa fractures. Mobility of the middle-third of the facial skeleton indicates a fracture of the Le Fort type (Fig. 3.5). Anterior or cribriform plate fractures or middle basilar skull fractures should be suspected when CSF rhinorrhea is present. Central nervous system injury is implied by paralysis of one or more of the cranial nerves, impaired consciousness, depressed sensorium, unequal pupillary size, extremity paralysis, abnormal neurological reflexes, convulsions, delirium, or irrational behavior.
Computerized tomographic scans
The definitive radiographic evaluation is the craniofacial CT scan with axial, coronal and sagittal sections of bone and soft tissue windows.5–7 However, the clinical examination remains the most sensitive detection of the character and functional implications of the facial injury.
CT evaluation of the face can define bone fractures, whereas, the soft tissue views allow for soft tissue definition of the area of the fracture. 3D CT Scans8–10 allow comparison of symmetry and volume of the two sides of the facial bones. Specialized views, such as those of the orbital apex, allow for a special magnified visualization. Fractures missed in CT scans are those in which there is little or no bony displacement or films where reading occurs from soft tissue views or thicker cuts.
Upper facial fractures
Frontal bone and sinus injury patterns
The frontal sinuses are paired structures that have only an ethmoidal anlage at birth. They have no frontal bone component initially. They begin to be detected at 3 years of age, but significant pneumatic expansion does not begin to occur until approximately age 7 years. The full development of the frontal sinuses is complete by the age of 18–20.11 The frontal sinuses are lined with respiratory epithelium, which consists of a ciliated membrane with mucus secreting glands. A blanket of mucin is essential for normal function and the cilia beat this mucin in the direction of the nasofrontal ducts. The exact function of the paranasal sinuses is still incompletely determined. When injured, they serve as a focus for infection, especially if duct function is impaired. Their structure, however, often protects the intracranial contents from injury by absorbing energy.
The predominant form of frontal sinus injury is fracture. Fracture involvement of the frontal sinus has been estimated to occur in 2–12% of all cranial fractures and severe fractures occur in 0.7–2% of patients with cranial or cerebral trauma.11
Approximately one-third of fractures involve the anterior table alone, and 60% involve the anterior table and posterior table and/or ducts. The remainder involves the posterior wall alone. Some 40% of frontal sinus fractures have an accompanying dural laceration.11
Clinical examination
Small fractures of the frontal sinus may be difficult to detect, especially if they are nondisplaced. Therefore, occasionally the first presentation of a frontal sinus fracture may be an infection or symptom of frontal sinus obstruction, such as mucocele, or abscess formation.12,13 Infection in the frontal sinus have the potential to cause significant morbidity due to its proximity to the brain location near the brain.14 Infections include meningitis, extradural or intradural abscess, intracranial abscess, osteomyelitis of the frontal bone, or osteitis in devitalized bone fragments.15–23
Nasofrontal duct
The development of a frontal sinus mucocele is linked to obstruction of the nasofrontal duct,1 which is involved with fractures in over nearly half of the cases of frontal sinus injury. The duct passes through the anterior ethmoidal air cells to exit adjacent to the ethmoidal infundibulum. Blockage of the nasofrontal duct prevents adequate drainage of the normal mucosal secretions and predisposes to the development of obstructive epithelial lined cysts or mucoceles. Mucoceles24–26 may also develop when islands of mucosa are trapped by scar tissue within fracture lines and attempt to grow after the injury producing a mucus membrane lined cystic structure which is obstructed.
The sinus is completely obliterated only when the duct is also deprived of its lining and when the bone is burred, eliminating the foramina of Breschet,27 in which it has been demonstrated that mucosal ingrowth occurs along veins in the walls of the sinuses.28,29 Regrowth of mucosa can also occur from any portion of the frontal sinus, especially if incompletely debrided. The reported average interval between the primary injury and development of frontal sinus mucocele is 7.5 years.
Surgical treatment
Frontal sinus fractures should be characterized by describing both the anatomic location of the fractures and their displacement. The indications for surgical intervention in frontal sinus fractures include depression of the anterior table, radiographic demonstration of involvement of the nasofrontal duct with presumed future nonfunction, obstruction of the duct with persistent air fluid levels, mucocele formation, and fractures of the posterior table that are displaced and presumably have lacerated the dura.30,31 Some authors recommend exploration of any posterior table fracture or any fracture in which an air fluid level is visible. Others have a more selective approach, exploring posterior wall fractures only if their displacement exceeds the width of the posterior table. This distance suggests simultaneous dural laceration. Simple linear fractures of the anterior and posterior sinus walls which are undisplaced are observed by many clinicians.
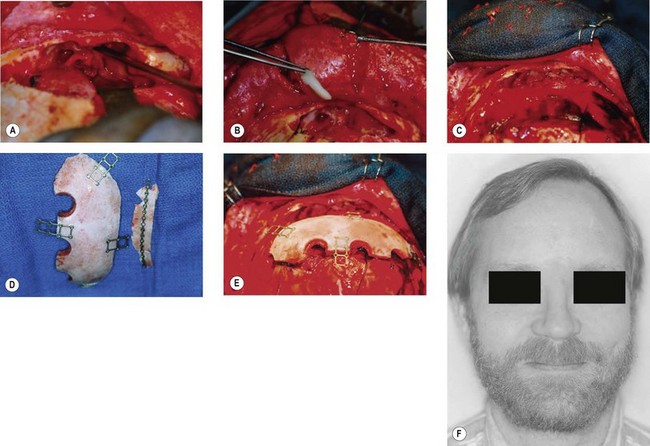
Any depressed frontal sinus fracture of the anterior wall potentially requires exploration and wall replacement in an anatomical position to prevent contour deformity. Most of these patients will have no compromise of nasofrontal duct function, however, some do and these should have the sinus defunctionalized. The anterior wall of the sinus may be explored by an appropriate local laceration or a coronal incision, or more recently endoscopically. Anterior wall fragments are elevated and plated into position. If it is desired that the nasofrontal duct32–34 and sinus be obliterated because of involvement, the mucosa is thoroughly stripped, even into the recesses of the sinus, and the nasofrontal ducts occluded with well-designed “formed-to-fit” calvarial bone plugs (Fig. 3.6). If most of the posterior bony wall is intact, the entire frontal sinus cavity may be filled either with fat or cancellous bone. The iliac crest provides a generous source of rich cancellous bone.35 Alternatively, the cavity may be left vacant, a process called “osteoneogenesis”. The cavity fills slowly with a combination of bone and fibrous tissue, but is more frequently infected in the authors’ experience than sinuses treated with cavity filling.36
If the posterior table is missing, no grafting need be performed for localized defects, but it is emphasized that the floor of the anterior cranial fossa should be reconstructed with bone. In cranialization, the posterior wall of the frontal sinus is removed, effectively making the frontal sinus a part of the intracranial cavity. The “dead space” may be filled with cancellous bone37 or left open. Any communication with the nose by the nasofrontal duct or with the ethmoid sinuses should be sealed with carefully designed bone grafts. The orbital roof should be reconstructed primarily by thin bone grafts placed external to the orbital cavity. An intracranial exposure is often required for this orbital roof reconstruction.
The use of a galeal flap38,39 in the treatment of extensive frontal bone defects designed with a pedicle of the superficial temporal artery can be a useful method for vascularized soft tissue obliteration of frontal bone problems.
Orbital fractures
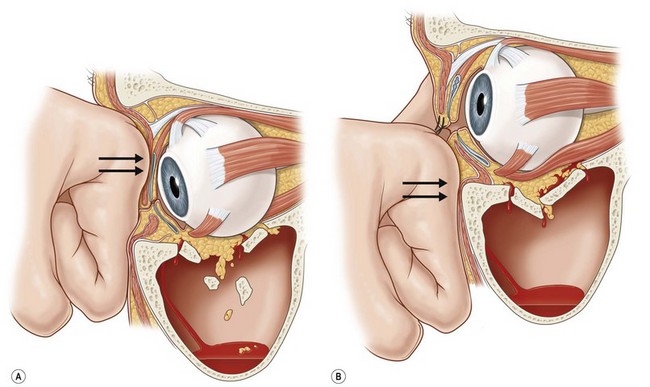
Orbital fractures may occur as isolated fractures of the internal orbit (also called “pure”) or may involve both the internal orbit and the orbital rim (also called “impure”) (Fig. 3.7).40–42
Orbital physical examination
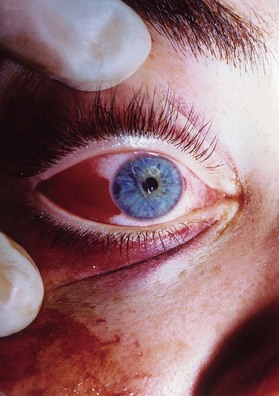
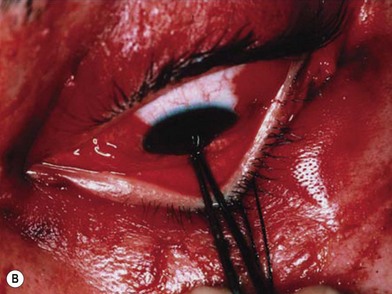
The examination should detect edema, corneal abrasion, laceration, contusion and hematoma. The simultaneous presence of a subconjunctival hematoma and a periorbital hematoma confined to the distribution of the orbital septum is evidence of a facial fracture involving the orbit until proven otherwise by radiographs (Fig. 3.8). The extraocular movements should note double vision or restricted globe movement. Visual acuity may be recorded by the patient’s ability to read newsprint or an ophthalmic examination card such as the Rosenbaum® Pocket card. Visual field examinations should be performed. All patients must be frequently checked for light perception and pupillary afferent defects preoperatively and postoperatively. Globe pressure may be assessed by tonometry and should be less than 15 mm. The results of a fundus examination should be recorded. The presence of no light perception indicates optic nerve damage or globe rupture. Light perception without usable vision indicates optic nerve damage, retinal detachment, hyphema, vitreous hemorrhage or anterior or posterior chamber injuries. Globe and eye injuries require expert ophthalmologic consultation.
Indications for surgical treatment
The indications for surgical treatment include:
• Double vision caused by incarceration of muscle or the fine ligament system, documented by forced duction examination and suggested by CT scans.
• Radiographic evidence of extensive fracture, such that enophthalmos would occur.
• Enophthalmos or exophthalmos (significant globe positional change) produced by an orbital volume change.
• Visual acuity deficit, increasing and not responsive to medical dose steroids, implying that optic canal decompression would be indicated.
• “Blow-in” orbital fractures that involve the medial or lateral walls of the orbit, and severely constrict orbital volume, creating increased intraorbital pressure.
Blow-out fractures in younger individuals
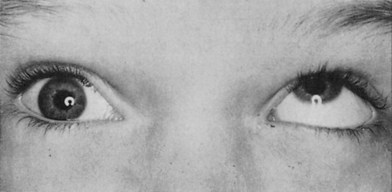
In children, the mechanism of entrapment is more frequently trapdoor than the “blow-out or punched out” fracture seen in adults. As opposed to incarceration of fat adjacent to the inferior rectus muscle, children more frequently “scissor” or capture the muscle directly in the fracture site. This may be suggested on physical examination with near immobility of the eye when upgaze is attempted on the affected side (Fig. 3.9). Trapdoor fractures with actual muscle incarceration are an urgent situation that demands immediate release of the incarcerated muscle.43–47 A number of authors have recently emphasized that a better prognosis is possible if the muscle is released early. The patient with true muscle entrapment may experience pain on attempted eye motion as well as nausea, vomiting, and an oculocardiac reflex,48 which consists of nausea, bradycardia, and hypotension.
Surgical treatment
The surgical treatment of orbital fractures has three goals:
• Disengage entrapped structures and restore ocular rotatory function.
• Replace orbital contents into the usual confines of the normal bony orbital cavity, including restoration of both orbital volume and shape.
• Restore orbital cavity walls, which in effect replaces the tissues into their proper position and dictates the shape into which the soft tissue can scar.
Operative technique for orbital fractures
Endoscopic approaches for orbital floor fractures
Recently, endoscopic approaches through the maxillary sinus have permitted direct visualization of the orbital floor and manipulation of the soft tissue and floor repair with this approach, which avoids an eyelid incision (Fig. 3.10).49–52
Cutaneous exposures
A number of incisions have been employed to approach the orbital floor:
• Lower eyelid incision. These have the least incidence of lower eyelid ectropion of any lid incision location but tend to be the most noticeable and prone to lymphedema.53–55
• Subciliary skin muscle flap incision. This incision near the upper margin of the lid leaves the least conspicuous cutaneous scar.56–58 However, they are prone to have the highest incidence of lid retraction.
• Transconjunctival incision. A preseptal or retroseptal dissection plane can be established.
Surgical treatment
Generally, a corneal protector is placed over the eye to protect the globe and cornea from instruments, retractors or rotating drills. The inferior rectus muscle, the orbital fat and any orbital soft tissue structures should be carefully dissected free from the areas of the blow-out fracture. Intact orbital floor must be located around all the edges of the displaced “blow-out” fracture. The floor must be explored sufficiently far back into the orbit that the posterior edge of the intact orbital floor beyond any defect can be identified. Many individuals call this “the ledge”, and it may be the orbital process of the palatine bone. Placing a freer periosteal elevator into the maxillary sinus and feeling the back of the sinus can verify the position of “the ledge”. The “ledge” is located just above the back of the maxillary sinus 35–38 mm behind the orbital rim. Identification of the intact “ledge” may be verified on CT scan (best shown on sagittal images).59,60 The “ledge” is the anatomical mark for which the implant material should be landed posteriorly to reestablish continuity of the orbital floor.
The forced duction test
Limitation of forced rotation of the eyeball is the “forced duction” test or the “eyeball traction” test (Fig. 3.11). This test provides a means of differentiating entrapment of the extraocular muscles from weakness, paralysis or contusion. The forced duction test should be performed: (1) before dissection; (2) after dissection; (3) after the insertion of each material used to reconstruct the orbital wall; (4) just prior to closure of the incisions. It is vital that these measurements be compared. It is absolutely imperative that any reconstructive material does not interfere with globe movement in any way, and it is absolutely essential to prove this before any incisions are closed. A full range of all oculo-rotatory movements must accompany restoration of proper eye position.
Restoration of continuity of the orbital floor
The purpose of the orbital floor replacement, whether a bone graft or an inorganic implant, is to reestablish the volume and the shape of the orbital cavity. This replaces the orbital soft tissue contents and allows scar tissue to form in an anatomic position (Fig. 3.12).
Bone grafts for orbital floor reconstruction
Split calvarial, iliac, or split rib bone grafts provide the ideal physiological substitute material for reconstruction of the internal orbital fractures.61,62 It is not known whether bone grafts resist bacterial colonization better than inorganic implants, but that is the presumption. Bone grafts are presumed to survive at the 50–80% level.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree