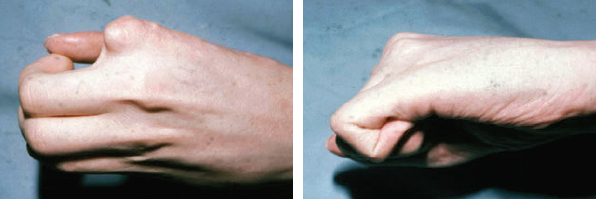
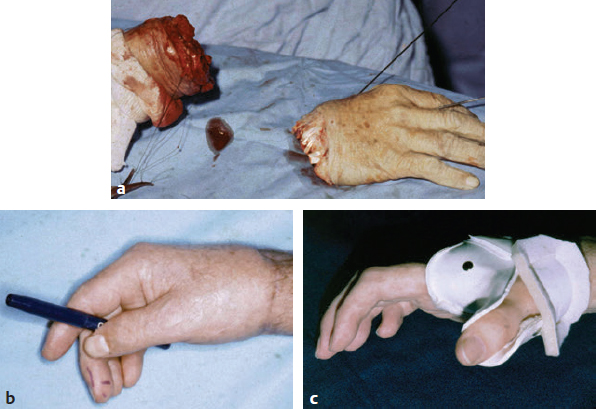
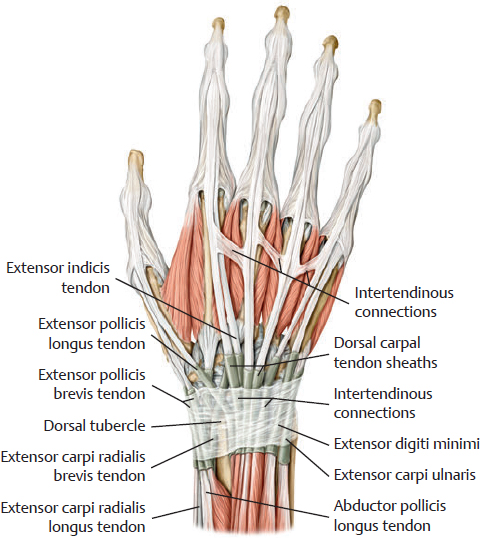
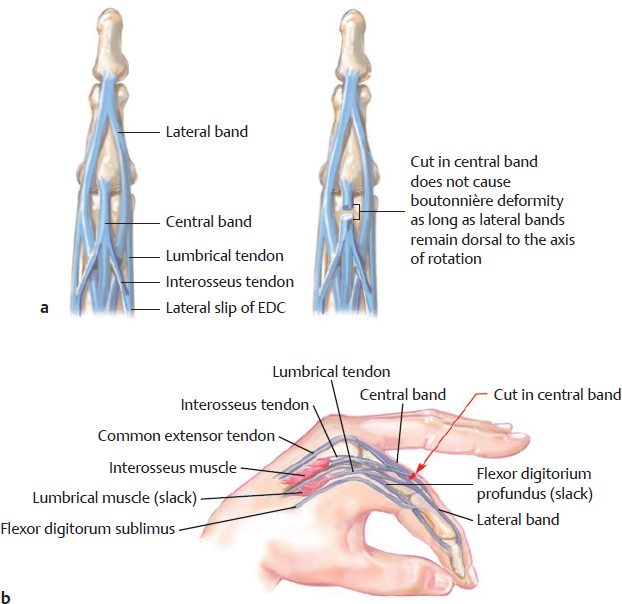
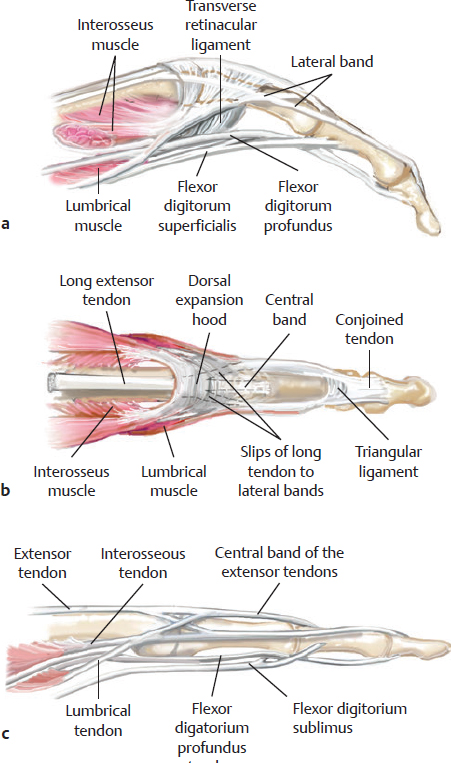
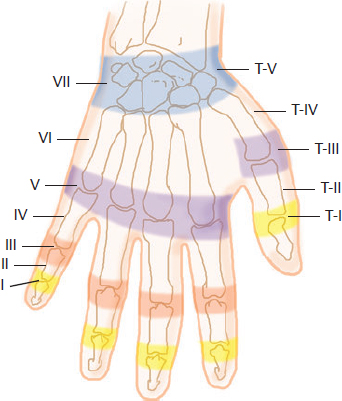
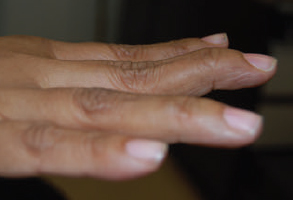
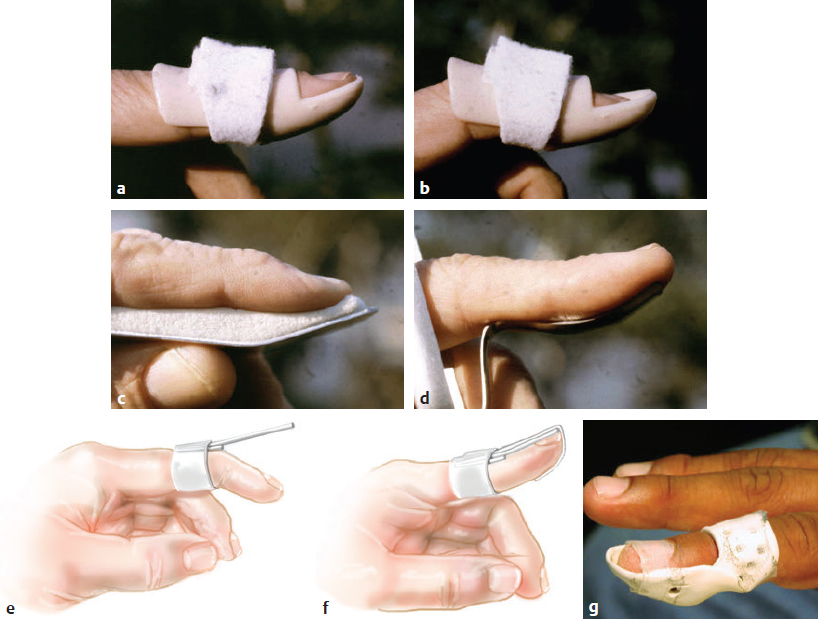
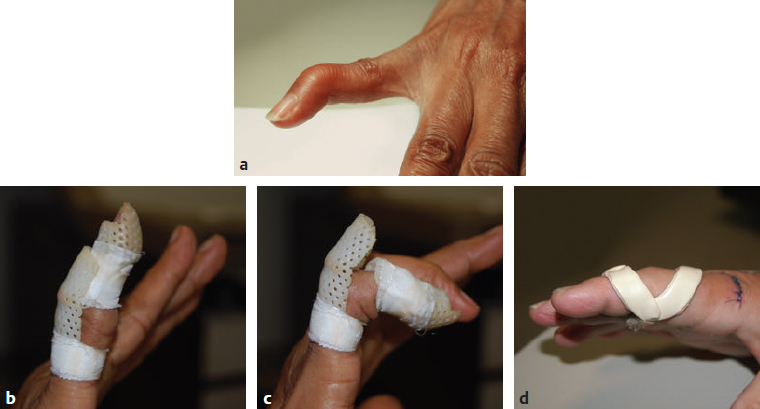
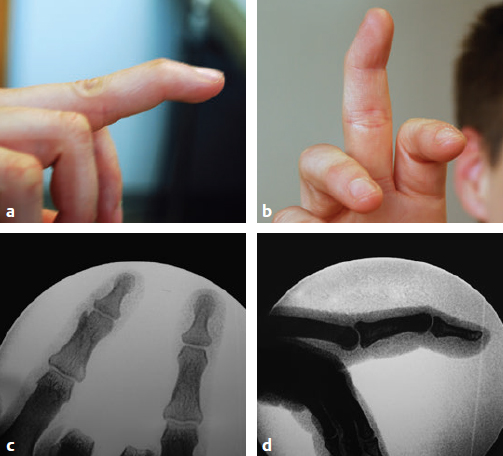
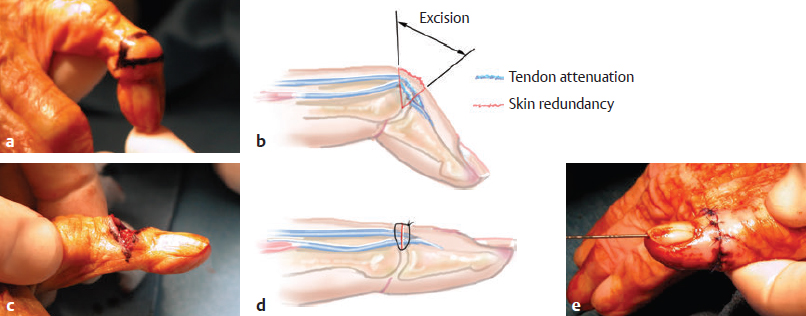
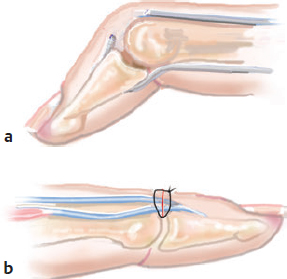
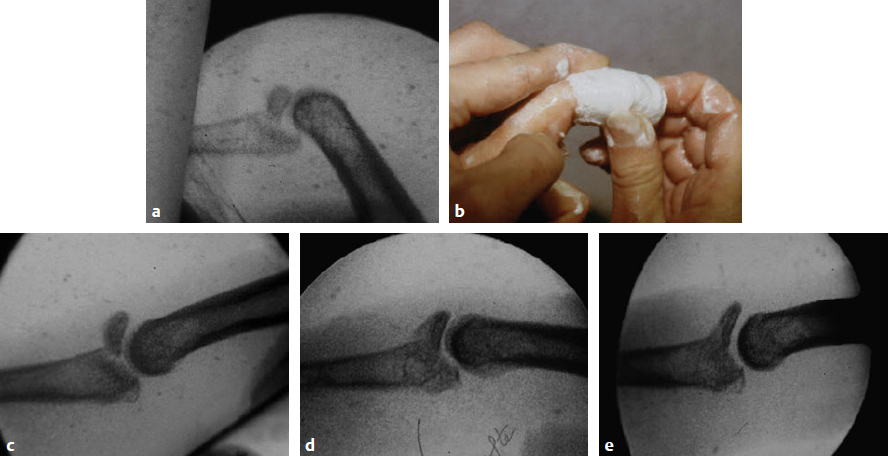
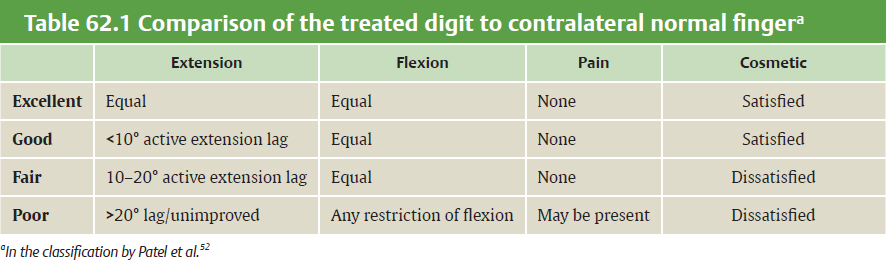
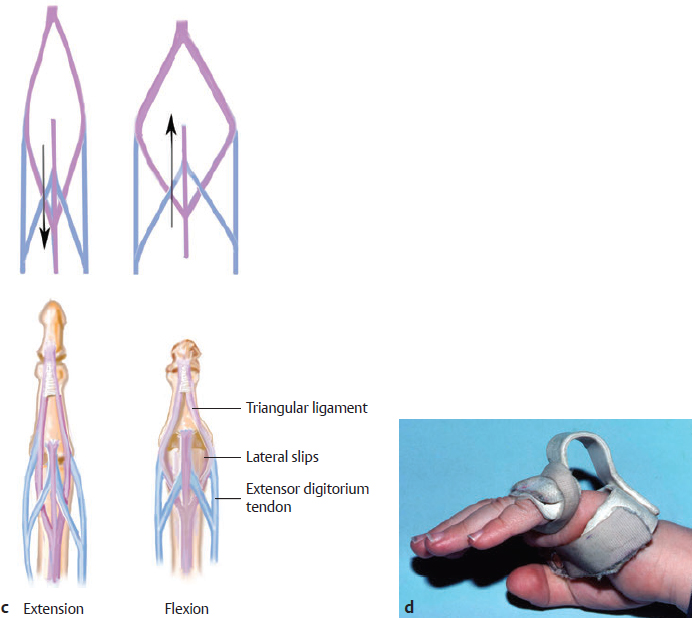
CHAPTER Extensor tendons are located superficially and thus are vulnerable to injury. Probably because surgical exposure is simple, these injuries receive much less attention in publications than do flexor tendons, yet morbidity is deceptively high. Unfavorable results are often related to loss of the delicate balance between the extrinsic and intrinsic extensor systems, further complicated by morbid biologic changes in adjacent uninjured structures proximate to the injured tendon itself. These lead to stiffness with changes in the volar plate, collateral ligaments, joint capsule, and even to adjacent uninjured digits. Acceptance of a less than favorable outcome has thus almost become the norm for many extensor tendon injuries, such as mallet and boutonnière deformity and rheumatoid tendon ruptures. We contend that this should not be the case. This chapter emphasizes a number of commonly overlooked management issues that lead to prevention of deteriorated function with adjacent, seemingly uninjured, structures, as well as addressing the injured tendon itself. The surgeon needs to have an understanding of certain biologic principles of healing and repair and of functional extensor tendon anatomy before exploring each zone of extensor tendon injury in its specific detail. Summary Box Unfavorable Results and Complications Associated with Extensor Tendon Repair • Adverse effects on adjacent structures • Fibroplasia • Reflex sympathetic dystrophy • Complex regional pain syndrome/reflex sympathetic dystrophy • Loss of flexion • Extensor tendon scar adhesions • Joint capsule extension contractures • Distal interphalangeal extension lag • Volar plate laxity • Volar plate contraction • General discomfort • Swan neck deformity • Extensor tendon rupture at the wrist, caput ulnae (Vaughan-Jackson) syndrome • Splint-induced complications • Collateral ligament contraction • Failure to achieve bone union • Ankylosed joint • Dorsal bump • Boutonnière deformity • Clinical repair rupture • Infection There are three important biologic principles that, if neglected, lead to unfavorable outcomes. 1. The hand should be considered as a “functioning organ,” because any injury results in a response by the entire organ.1 2. A challenge in hand surgery, with all types of injuries, is to preserve function in all structures that may not have been directly injured.2 3. Scar will most simulate the tissue to which it is juxtaposed1 (the “one wound, one scar” enunciated by Peacock3). An unfavorable result after extensor injuries commonly results from adverse effects on adjacent structures that are not necessarily directly injured (Fig. 62.1). It is a misconception that structures composed of connective tissue (tendon, ligament, fascia, and the joint capsule) are biologically inactive. Connective tissue develops a dramatic increase in collagen synthesis and turnover, even greater than in skin wounds, and continues at this level of activity long after a similar skin injury would.2 This explains why swelling and inflammation in the injured digit might continue for months and why therapy can modify outcomes weeks or even months after injury as long as it remains inflamed. This is because the accelerated collagen turnover results in a high level of uncross-linked collagen fibers that can be modified in response to stretch stresses. Activated macrophages in an injured digit may equally involve the site of tendon laceration and the adjacent intact collateral ligament or volar plate, resulting in fibroplasia. Structures that are closest to the site of injury undergo the greatest change, but even the entire hand can respond with swelling and generalized stiffness, seen at its most dramatic with reflex sympathetic dystrophy and complex regional pain syndrome. As with any other tissues, tendon healing occurs in three phases: (1) inflammatory, (2) proliferative, and (3) remodeling.4 During the inflammatory phase, activated macrophages target necrotic tissue and release chemoattractants, drawing in tenocytes.5 The proliferative phase results from tenocyte–macrophage collagen synthesis, starting several days after injury. It is thus best to rest most injured hands in a splint and bulky dressings for 3 to 5 days while surgical trauma subsides and fibroblasts are mobilized. Initially, type III collagen is deposited, but later type I collagen predominates and the extracellular matrix becomes more organized, cross-linked, and less responsive to therapy stretch. This remodeling phase may continue for up to 1 year, and repaired tendons never regain their original strength.6 Mechanical stress has a direct effect on tendon healing. Too much stress can disrupt a healing tendon, whereas early, protected motion results in increased vascularity and deoxyribonucleic acid (DNA) synthesis and overgrowth of epitenon at the repair site, which is a phenomenon not seen in immobilized healing tendons. This results in both increased tensile strength and improved gliding function.7 Early active motion also allows tendons to heal with fewer extrinsic adhesions.8 Although these observations are noted most in studies on flexor tendons, they apply equally to extensor tendons. These principles of protected early motion have not been as rigorously applied to healing extensor tendons, perhaps accounting for less well-studied outcomes in extensor tendon injury. The surgeon must focus not only on the healing tendon, but also on the adverse response to injury of seemingly uninjured adjacent structures9 (Fig. 62.2). This is best accomplished by early motion in such a way that repair is not disrupted, emphasizing rigid fracture fixation and a protected tendon motion regimen, such as rubber band active dynamic traction, short-arc controlled motion, and relative-motion splinting.9,10 Relative-motion splinting allows early protected active motion and minimally inhibits functional use of the hand after repair of long extensor tendons, boutonnière deformity, and sagittal band rupture. We also advocate use of local anesthesia with epinephrine and no tourniquet during surgical repair to ensure that the correct extensor mechanism balance is restored and to verify by direct intraoperative observation the protection afforded by the relative-motion orthosis.11 Fig. 62.1 This patient sustained an avulsion amputation of the index finger. Swelling and lack of use resulted in stiffness of the remaining fingers with metacarpophalangeal joint extension contractures, even as distant from the amputation site as the little finger. Fig. 62.2 (a) This traumatically amputated hand was replanted. (b) The long-term result had a thumb adduction contracture, which could possibly have been prevented by (c) a protective thumb web splint. (Reproduced from Merritt WH. Written on behalf of the stiff finger. J Hand Ther 1998;11(2):74–79.) There is a prolonged increased collagen turnover in structures made of dense connective tissue beyond the time taken for skin wounds to have matured. This collagen response is greatest along the surfaces of the structures rather than within their deep substance. As much as a 20-fold collagen increase may occur in the thin areolar surface of the fascia within 5 days of simple laceration.10,12 This creates a “coat” of collagen that adheres to skin and affects the mobility of the collateral ligament, joint capsule, muscle fascia, and tendon once that added collagen has become cross-linked.9 The most common unfavorable result of long extensor tendon repairs that are immobilized postoperatively is loss of flexion and not of extension, most likely related to remodeling of the uninjured joint capsule collagen and the collateral ligaments.13 This complication can be minimized by one of the early motion protocols that have been espoused, three of which have been most studied and popularized to include relative-motion extensor splinting,14–16 active short-arc motion,9 and early passive motion with dynamic extension outrigger splinting.17 The knowledge and skills of a hand therapist are essential for a favorable outcome. Peacock3 pointed out that after injury, a single viscous uniform scar develops between all injured collagen structures to the full depth of the wound—bone, tendon, paratenon, tendon sheath, and skin. Scars cannot regenerate into the identical original biologic structures, but will show some structural similarity to the adjacent tissue. Thus rough surgical handling with loss of paratenon (the important tendon gliding surface) may result in tendon scar adhesions.18 Likewise, a complex crushing injury with raw bone surface and muscle denuded of dense connective tissue will result in an “unfavorable scar” for gliding. Early protective motion tends to reduce cross-linking of adhesive collagen in unfavorable scars and can produce a more favorable gliding surface. On occasion, the surgeon may choose to interpose a gliding surface for tendons, such as a vascularized fascial flap, or create a more favorable scar bed with a silicone rod spacer. Coordinated functional hand movement requires a balance not only between long flexor and extensor tendons, but also between the extrinsic and intrinsic extensor mechanism. In addition, movement at one joint is affected by the relative position of adjacent joints; for example, with the wrist extended and stabilized, digital extensors are lax and relaxed, enabling stronger finger flexion, which is the position of grip strength.19 With the relative tenodesis across the wrist, finger flexion is more difficult when the wrist is flexed. This relative motion becomes important when a number of tendons have a common muscle belly, as is the case for both the extensor digitorum communis and flexor digitorum profundus. Because of the common muscle origin, metacarpophalangeal (MCP) joint positioning in one finger will affect adjacent fingers. This permits early active motion by providing protection from a relative-motion splint.20 The extrinsic extensor muscles can be divided into superficial and deep layers. The superficial layer consists of, in order of their innervation from proximal to distal: • Extensor carpi radialis longus (ECRL) • Extensor carpi radialis brevis (ECRB) • Extensor digitorum communis (EDC) • Extensor digiti minimi (EDM) • Extensor carpi ulnaris (ECU) The ECRL, ECRB, and EDC originate from the lateral epicondyle of the humerus and insert on the base of the second metacarpal, base of the third metacarpal, and individual finger extensor mechanisms, respectively. The EDM and ECU originate below the elbow joint and insert into the small finger extensor mechanism and the base of the fifth metacarpal, respectively.21 The deep layer muscles originate further distally along the interosseous membrane, consisting of, again in order of their proximal to distal innervation: • Abductor pollicis longus (APL) • Extensor pollicis brevis (EPB) • Extensor pollicis longus (EPL) • Extensor indicis proprius (EIP) The APL, EPB, and EPL insert on the base of the third metacarpal, the base of the thumb proximal phalanx, and the base of the thumb distal phalanx, respectively. The EIP in serts into the extensor mechanism of the index finger, usually found ulnar to the EDC index insertion. The extensor muscles of the superficial and deep layers are innervated by the posterior interosseous nerve (PIN), with the exception of ECRL, which is innervated before the take-off of the PIN.22 As the tendons of the extensor muscles traverse the wrist, they are contained within six extensor compartments, numbered progressively from radial to ulnar (Fig. 62.3). The first extensor compartment, which runs along the radial styloid, contains APL and EPB. A septum may be present between the two tendons, and has been reported to occur in 34% to 77.5% of cadaver specimens.23,24 When overlooked, this structure can be responsible for a failure of surgical release of de Quervain’s tenosynovitis. The second extensor compartment contains ECRL and ECRB, which is separated from the third compartment by Lister’s tubercle, which has the EPL passing around its ulnar border before entering the third compartment. Just proximal to the extensor retinaculum, the musculotendinous junctions of the APL and EPB cross over the radial wrist extensors. Pain and swelling in this area can be caused by stenosing tenosynovitis, referred to as “intersection syndrome.”25 The fourth extensor compartment contains EIP and EDC. The fifth compartment, which lies dorsal to the distal radioulnar joint (DRUJ), contains EDM, and the sixth compartment contains ECU. There is much variation in extensor tendon anatomy, especially between the retinaculum and the MCP joints. The EDC, EIP, and EDM tendons may have multiple longitudinal slips. The tendons of the EIP, EDC-index, and EDC-long are usually single-tendon units. The EDC-ring has two or three slips 61% of the time, and the EDC-small is absent in 54% of cadaver specimens. The EDM usually has two slips and can even have a slip that inserts on the ring finger extensor mechanism in rare cases.26 There are many variations of extensor anatomy, so the surgeon must be cautious when evaluating these injuries and never assume the anatomy will follow a specific textbook description. Fig. 62.3 Anatomy of the extensor tendons over the dorsum of the hand, in their synovial compartments at the wrist, and in the forearm. (Reproduced from Gilroy AM, MacPherson BR. Atlas of Anatomy. 3rd ed. New York: Thieme Medical; 2017.) The juncturae tendinum are dense connecting bands from one extensor tendon to another. They form between adjacent tendons and so may mask a complete tendon laceration, because an adjacent tendon may pull on the distal lacerated tendon end and still effect finger extension. They may connect the common extensors of the EDC as well as the EDC-ring to the EDM. The EIP does not have juncturae.27 There are three subtypes of juncturae based on their thickness28: 1. Type I is thin and filamentous. 2. Type II is thicker. 3. Type III has well-defined tendon slips that are thick enough to be used as tendon grafts for pulley reconstruction.14 There are wide variations in junctural anatomy. In 54% of patients, the EDC to the small finger is absent and then is represented by a type III junctura between the EDC-ring and EDM.14 At the MCP joint, the extensor mechanism fans out to form a broad hood (Fig. 62.4). At the MCP level, the sagittal band on either side of the central extensor tendon blends volarly with the volar plate and flexor tendon sheath.29 This creates a yoke around the base of the proximal phalanx and serves as the mechanism by which the MCP joint extends, because there is no direct insertion of the extensor mechanism to the dorsum of the proximal phalanx. Sagittal bands centralize and stabilize the EDC over the MCP joint, so sagittal band rupture causes EDC tendon subluxation into the adjacent web space, which results in MCP joint extension lag. The long extensor trifurcates proximal to the proximal interphalangeal (PIP) level into a central portion and two lateral portions, which blend into the lateral band proximal to the PIP joint (see Fig. 62.4a). Distal to the PIP joint, the triangular ligament attaches to the two lateral bands (see Fig. 62.4c) and so stabilizes them in place. The lateral bands separate normally during PIP flexion but are restricted from too much lateral movement by the triangular Winslow’s ligament, encircling the PIP joint and opening and closing with flexion and extension, respectively, known as “Winslow’s diamond” (see Fig. 62.4c).30 Separation and volar positioning of the lateral band below the PIP axis of rotation is necessary for a boutonnière deformity to develop. Not only is the central slip disrupted, but there is also rupture or attenuation of the triangular ligament and archiform hood fibers. This explains why it sometimes takes time after a closed boutonnière injury for the full deformity to develop. It also explains why the Fowler procedure, which creates an isolated division of the extensor central slip to reduce a swan neck deformity, does not cause a boutonnière problem, and PIP extension is preserved through the intact triangular ligament and transverse and oblique fibers of the extensor hood.31 An understanding of this extensor anatomy of the PIP joint is critical to elucidating why relative motion flexor splinting may be successful for boutonnière deformities.9 When the MCP joint is placed in a greater relative flexion than adjacent digits, greater tension is then exerted on the long extensor and its effect on the interphalangeal joints through the intact lateral bands (see Fig. 62.4b). Similarly, blocking the MCP joint in the intrinsic paralyzed hand allows IP extension in the fourth and fifth digits when the long extensors are activated (see Fig. 62.4d). Fig. 62.4 (a) Dorsal and lateral views of the extensor apparatus of the finger. The central long extensor trifurcates into the midline central slip and lateral contributions to the lateral bands. (b) Insertions of the extensor mechanism: Winslow’s diamond encircles the proximal interphalangeal joint, central slip, and lateral bands into a conjoined terminal tendon. (c) Winslow’s diamond. (d) Even if central slip is disrupted, or if there is intrinsic palsy from ulnar nerve injury, blocking of the MCP joint will allow interphalangeal extension through the extrinsic–intrinsic interconnections of the lateral slips. As seen previously, the long (extrinsic) and intrinsic extensors are intimately associated as they blend over the extensor hood of the digits. The intrinsic muscles join into this extensor mechanism at the level of the MCP joint (where they are the prime flexors through the transverse fibers), although they are principally IP extenders in the extensor mechanism. The lumbrical muscles originate from the radial side of the flexor digitorum profundus (FDP) to each finger proximal to the A1 pulley. Their tendons cross volar to the transverse metacarpal ligament. Lumbricals, interossei, and the long extensors (through their lateral trifurcations) all contribute fibers to form the radial lateral band. Thus lumbrical tendons cross volar to the MCP joint axis of rotation and functionally flex that joint. Distally, the lateral bands extend dorsal to the PIP joint axis of rotation and continue on to the DIP joint, resulting in both PIP and DIP extension. With the PIP joint fully flexed, the DIP joint cannot actively extend because of relaxation of the intrinsic mechanism due to being tethered by the central slip insertion, unless the lateral bands are freed from the central slip insertion and pathologically displaced volar (Elson)32 (see Fig. 62.18e, later in the chapter). The median nerve innervates the lumbricals through the index and long fingers, and the lumbricals to the ring and small finger are innervated by the ulnar nerve (with the respective innervations to the FDP tendon to those digits). There are two rows of interosseous muscle, dorsal and palmar. Four dorsal interossei abduct the index, long, and ring fingers. Three palmar interossei adduct the index, ring, and small fingers. All interosseous muscles originate from the metacarpal of the finger on which they insert and receive innervation from the deep motor branch of the ulnar nerve and function as flexors of the MCP joint. Their tendons pass dorsal to the transverse metacarpal ligament and, in concert with the lumbricals, extend the IP joint. Tendons both insert on the base of the proximal phalanges to abduct or adduct the finger and send fibers to the lateral bands, which contribute to extension of the PIP and DIP joint.33 As the long extensor courses dorsal to the proximal phalanx, it trifurcates, sending lateral branches to the radial and ulnar side of the digit, contributing to the lateral bands, and thus permitting interphalangeal extension by relative-motion flexion splinting for correction of a boutonnière deformity. The lateral bands then continue distally. They are joined together by the triangular ligament just distal to the PIP joint. Lateral bands then join dorsally to form the conjoined tendon, which inserts on the dorsal base of the distal phalanx. The dorsal positioning of the lateral bands to the axis of rotation of the PIP joint is critical to normal finger extension. If these lateral bands slip volar to the axis of rotation, they then flex the PIP joint while continuing to extend the DIP joint, resulting in a boutonnière deformity. The triangular Winslow’s ligament serves to prevent volar subluxation of lateral bands that cause boutonnière deformity, while the transverse retinacular ligament prevents dorsal subluxation by anchoring the lateral band to the flexor sheath at the level of the PIP joint, preventing dorsal migration of those lateral bands and thus preventing a swan neck deformity30 (Fig. 62.5). Landsmeer’s oblique retinacular ligaments (ORL) are the final extensor mechanism and have uncertain purpose. They extend proximally from each side of the distal conjoined tendon, passing volar and proximally to the axis of the PIP joint, where they attach into the lateral aspect of the proximal phalanx and flexor tendon sheath. Their functional significance has been debated. Landsmeer suggested a tenodesis effect that results in synchronized flexion and extension of the PIP and DIP joints.34 For example, with the PIP joint held in full extension, the DIP joint can typically only passively or actively flex to 45 degrees or maximally to 60 degrees. However, with the PIP joint in flexion and the ORL relaxed, 90 degrees of flexion at the DIP joint is easily permitted. Landsmeer also suggested that the extension of the DIP joint from a fully flexed position was initiated as a result of ORL tenodesis in response to the PIP joint extension, which pulls the ORL taut. However, several authors have argued that the ORL is not involved in active finger motion.35,36 Fig. 62.5 Anatomy of the extensor mechanism of the finger. (a,b) The “restraining ligaments”: the triangular ligament, transverse retinacular ligament, and oblique retinacular ligament. (c) The delicate interweaving of the contributions of both intrinsic and extrinsic systems, showing the extensor digitorum communis lateral slip contribution into the intrinsic system. The first congress of the International Federation of Societies for Surgery of the Hand reached a consensus regarding anatomical classification of flexor and extensor tendon injuries in 1980.37 The extensor system was divided into nine zones. In the fingers, extensor zones are numbered starting distally at the DIP joint. Moving proximally, each zone is numbered according to its relationship to the underlying joint or phalangeal bone. Proximal to the MCP joint, extensor zones are delineated by the hand dorsum and the extensor retinaculum, whereas the forearm is divided into distal and proximal segments. The thumb extensors have a unique numbering system because the thumb has only one IP joint (Fig. 62.6). Fig. 62.6 Extensor tendon zones of injury dorsal to (I) the distal interphalangeal joints, (II) the middle phalanx, (III) proximal interphalangeal joint, (IV) the proximal phalanx, (V) the metacarpophalangeal (MCP) joint, (VI) the hand, (VII) wrist (under the extensor retinaculum), (VIII) the distal forearm, and (IX) the midforearm and proximal forearm. These differ slightly for the thumb, which includes (T-I) an interphalangeal joint, (T-II) the proximal phalanx, (T-III) the MCP joint, (T-IV) the first metacarpal, and (T-V) the area between the carpometacarpal joint and radial styloid. A mallet deformity is the most common extensor tendon injury and perhaps the most consistently undertreated. Many suggest that even with treatment, there will be some mild residual extension lag.38 The injury occurs when the extended DIP joint is forcibly flexed, as with a jamming baseball injury. There is a rupture of the distal conjoined tendon or an avulsion fracture of the dorsal base of the distal phalanx and, in rare instances, both.39 The intrinsic imbalance leads to a hyperextension force transmitted through the PIP joint, and the DIP joint droops, so the deformity resembles a mallet tool (Fig. 62.7). The surgeon cannot be too casual in the treatment of these injuries. Even without severe DIP extension lag, some patients have inherent volar plate laxity at the PIP joint, so secondary PIP joint hyperextension and discomfort may develop. An unfavorable result from a mallet deformity is commonly not from the annoyance of the loss of DIP extension but from the resultant swan neck deformity, leading to difficulty initiating PIP joint flexion and ultimately to that joint becoming “locked” in extension. Overwhelmingly, there is support for nonoperative over surgical treatment for closed mallet deformities. This is largely related to the complications of each form of treatment. The reported experience in the treatment of 120 mallet fingers revealed that surgical repair had a 53% complication rate, compared with 45% when treated with splinting.40 However, splint-induced complications were nearly always transient, whereas 76% of surgical complications were still problematic at 38 months follow-up. Thus nonsurgical over surgical treatment can certainly help prevent potential complications. Even complications that result from splinting can probably be avoided by taking extra care in the approach to these patients. One potential reason for problems is the misconception that easily available commercial splints will fit any finger. An ill-fitting Stack splint may be too tight and cause dorsal skin pressure necrosis. Additionally, many Stack splints and padded aluminum splints will not maintain even a neutral DIP position, because they may fit too loosely (Fig. 62.8). Fig. 62.7 Chronic mallet deformity of DIP joint of middle finger has led to a dysfunctional swan neck problem. Fig. 62.8 (a) A properly fitted Stack splint. (b) An improperly fitting Stack splint that is too large and has obvious (though partially disguised by the splint) persistent flexion at the distal interphalangeal (DIP) joint. (c) An aluminum foam splint may be placed volar, but if the foam is retained in the splint, the fingertip sinks into the foam, allowing DIP flexion even if the metal is bent into extension. (d) If the foam is cut away, it allows a small amount of desirable DIP joint hyperextension. (e) A method of taping on a dorsally fitting aluminum foam splint. (f) Because Stack splints are premade, they do not always fit precisely. (g) A custom-made thermoplastic splint fits better and allows slight DIP joint hyperextension. The best extension that can be expected is that which can be achieved during splinting. Poor outcome and mismanagement may be related to poor choice of a commercial splint size, inadequate monitoring or miscommunication, and patient noncompliance. For example, it is common for a patient to insist on having been compliant with splinting and yet “removed the splint only when showering,” or for a patient to return with a Stack splint that has inadvertently been replaced upside down (Fig. 62.9). One potential disadvantage of the Stack splint is that the plastic covers the tactile volar distal finger pulp. It takes only 1.5 mm of tendon laxity or redundancy to create a 10-degree extension DIP lag.41 It is essential to individualize patient management because of the significant differences in DIP normal range of motion among patients. Observation of the patient’s normal side suggests the amount of hyperextension that should be used for immobilization. Fig. 62.9 (a) This patient returned to the office with the Stack splint mistakenly applied upside down. (b) The mallet deformity persisted because of the ineffective splinting. The initial presentation in the emergency room may be of a deceptively minor injury. A laceration of the DIP joint may initially involve a partial extensor tendon laceration that later propagates into a complete tendon disruption. Any suspicious laceration should be explored (using local anesthesia with epinephrine or with digital tourniquet), and any finger that is suspected to have an extensor tendon injury should be protected with a finger extensor orthosis. Dorsal DIP swelling from a closed injury may initially disguise the extension lag of the mallet disruption. Furthermore, it takes time for the imbalance to manifest between the extrinsic and intrinsic systems and for the full-blown swan neck problem to develop. All closed mallet injuries should be radiographed. Approximately one third will have a distal phalangeal fracture.42 Even if the results of the radiograph do not alter management, patients may “feel betrayed” to later discover they had a previously undiagnosed fracture. A true lateral finger radiograph will demonstrate whether the fracture is associated with a volar subluxated joint (Fig. 42.10). It is important to assess the degree of innate DIP and PIP hyperextension on the opposite, unaffected digit. Rather than trying to fit a prefabricated Stack splint at the time of acute injury when there is dorsal DIP swelling, it is better to custom design a thermoplastic splint with some DIP hyperextension. However, the surgeon must be cautious with excessive hyperextension or dorsal finger contact pressure, which could result in dorsal finger soft tissue necrosis. When seen in follow-up shortly thereafter, there may be 10 to 15 degrees of DIP flexion within the splint because loosening has occurred as swelling resolved. Adjustment will be required. Initially, it may be wise to include the PIP joint in the splint. This may need to be continued for patients who have innate PIP hyperextension laxity. This does not necessarily mean total immobilization of the PIP joint but merely blocking hyperextension, which can be done by adding, for example, a figure-of-eight PIP orthosis (Fig. 62.11). This is especially important for patients with delayed treatment and secondary PIP hyperextension. They will not recover full DIP extension while the PIP remains in abnormal hyperextension. Fig. 62.10 Mallet fracture with joint subluxation in (a) the extended view and more obvious in (b) the flexed lateral view. Fig. 62.11 (a) Some patients with pronounced proximal interphalangeal (PIP) hyperextension and mallet deformity need both a mallet splint and a dorsal PIP-blocking splint. (b) A finger in PIP extension. (c) A flexed finger. (d) The PIP joint hyperextension can also be controlled by a figure-of-eight splint. Fig. 62.12 (a,b) This patient had a swan neck deformity with a mallet droop to the distal interphalangeal joint. (c) This problem is a result of middle phalangeal foreshortening and extensor mechanism laxity from an oblique fracture malunion. (d) Lateral radiographs showed proximal interphalangeal hyperextension. (This patient was treated by reefing the conjoined tendon.) Finally, with regarding clinical assessment, a mallet injury should not be confused with a DIP extension lag that may occur from tendon imbalance that results from subsidence of a middle phalangeal angulated fracture (Fig. 62.12). The mallet treatment would revolve around the most appropriate treatment for the phalangeal fracture itself or reefing the lax extensor mechanism. There is much debate regarding the most appropriate orthosis for treatment of an acute closed mallet injury. A controlled, randomized trial found no significant differences between groups who had a Stack splint, dorsal two-part foam orthosis with a buckle over the DIP (“Mexican hat” orthosis), or custom-made circumferential thermoplastic (“thimble”) orthosis.43 These authors evaluated persistent extension lag, patient satisfaction, and reported pain after 8 weeks of splinting. There were fewer complications with the thermoplastic splint. Another study compared 12 weeks of splinting using a dorsal padded aluminum orthosis, volar padded aluminum orthosis, or custom thermoplastic orthosis.44 In all groups, there was a resultant final extension lag ranging from 9 to 16 degrees but no significant difference between the groups. Most reports recommend 6 to 10 weeks of extension splinting with slight hyperextension of the DIP joint, followed by 3 to 6 weeks of additional nighttime splinting, even though a randomized trial of night splinting did not seem to alter final outcomes.45 If the patient can lift the DIP joint from a flat table position, and all dorsal DIP swelling and erythema has resolved, the tendon is healed.46 Our treat ment regimen makes intuitive sense: We immobilize the DIP joint continuously, usually with some degree of hyperextension, based on the normal hyperextension of the opposite side. After 6 weeks, there is gradual daytime splint weaning and the splint is worn during all vigorous activities for an additional 2 to 3 weeks, with nighttime splinting during that additional 3 weeks. Mechanical stress across the healing tendon site strengthens that healing tendon, but in its recently healed but not fully strengthened state, full activity could lead to attenuation or rupture. Thus gradual resumption of stress is warranted. If any extensor lag develops, full splinting is again instituted and often will correct the lag. If the patient is unreliable, a longer duration of splinting or even supplemental K-wire fixation may be instituted. Finally, regarding management, temporary internal K-wire DIP immobilization has been suggested, but at least one study has compared K-wire immobilization to an extension external orthosis in acute injuries treated within 2 weeks, with no significant outcome differences.47–49 In certain patients, the additional potential morbidity of a .045 K-wire transarticular fixation, burying the wire beneath the skin, may be justified and affords the possibility to wear surgical gloves and to continue activity. An additional external orthosis is recommended when ungloved. Tubiana50 recommended placing the K-wire obliquely across the joint to minimize discomfort in the volar pulp and so that the wire can be retrieved if it migrates proximally. Patients may present long after the acute 2-week interval since injury, which is the preferred time within which to initiate splinting. This is because they initially misdiagnose the injury as a “sprain” or think that the injury may resolve on its own. Several reports give some comfort in nonetheless treating patients who present later with splinting. One report found no more than 10 degrees of DIP extension lag in 81% of 14 patients treated by orthosis within 2 weeks of closed injury, compared with 80% of patients treated more than 40 weeks after injury.51 There are a number of other examples of successful late treatment by splinting even with a 4-month or longer interval after injury.52,53 Success with late splinting is more likely when there is still evidence of potential tendon healing, evidenced by persistent pain, swelling, and erythema and other signs of an ongoing inflammatory response, regardless of the delay since injury. In contrast, a patient with an asymptomatic late mallet deformity who has no symptoms of inflammation is less likely to remodel an already-attenuated but mature scar at the site of tendon rupture. Patients must understand that the outcome of treatment if delayed beyond 6 to 8 weeks after injury is less predictable, especially if signs of ongoing inflammation have resolved. However, an attempt at closed splinting management is preferred. Fig. 62.13 (a,b) This 70-year-old with chronic mallet deformity had both skin and extensor tendon redundancy. (c,d) Redundant skin was excised elliptically, and dermatotenodesis sutures were placed. (e) K-wire fixation was then used for 6 weeks, followed by immobilization. When splinting has failed, the surgeon must resort to different treatment options. Patients with only minor DIP extension lag and no problems with PIP hyperextension may be left untreated. If the patient is bothered only by compensatory PIP hyperextension and difficulty with the mechanics of finger flexion, careful division of the central long extensor attachment to the middle phalanx will redistribute the balance between extrinsic and intrinsic tendon systems, allowing greater tension along the lateral bands transmitted to the distal phalanx and releasing the overpull on the central extensor tendon (as described by Fowler31). This operation requires a careful approach beneath the extensor hood, preserving the triangular ligament attachment between the lateral bands, or else a boutonnière deformity will develop.54 The Fowler procedure should not be considered before at least 6 to 12 months after injury to allow for a sufficiently mature scar at the DIP that is strong enough to redistribute forces that will extend the DIP joint. Furthermore, some patients spontaneously develop a sufficient degree of improvement without surgery during that interval.55 Patients who present with a chronic mallet deformity and who still desire definitive treatment or who have had a splinting failure may potentially benefit from a surgical tenodermatodesis (Fig. 62.13). This re-creates the original defect by skin and tendon scar excision followed by immobilization, usually with K-wire transarticular fixation and an additional external orthosis. This technique was described by Iselin et al.56 Provided that the DIP joint can be passively extended, we have seen this procedure succeed as long as 20 years after the original injury. We prefer to place the K-wire at the tip of the digit and leave it exposed where it can be protected by a custom thermoplastic orthosis. This not only facilitates later removal but also discourages premature patient removal of the splint. A simple surgical solution for the patient with a very pronounced residual extension lag at the DIP joint may be to perform an arthrodesis at the DIP joint. However, this results in fusion of an essentially normal joint. The obvious indication for surgical repair is an already open injury. However, there is some controversy regarding this injury. McFarlane and Hampole57 recommended closing both skin and tendon in a single figure-of-eight suture layer, if repair is done at all, and thereafter managing in the same manner as that used for a closed injury with external splinting. However, they also felt that if the tendon repair would prove too flimsy, they would dress the skin wound only, with the DIP joint immobilized in slight hyperextension. The results in these patients were no different in open or closed injuries. Doyle58 also agrees with this approach. The surgeon might not use braided sutures if the tendon is repaired and should use a single-suture technique to reduce infection complications.59 One other report recommended 5–0 nylon for tendon repair or 4–0 continuous monofilament or wire pullout (if there is inadequate distal tendon stump for repair) with separate skin closure, along with a transarticular K-wire fixing the DIP joint in slight hyperextension, placed before the tendon repair.60 Our approach has been similar, relying on an initial placement of a transarticular longitudinal wire with the DIP joint in slight hyperextension, followed by repair of skin and tendon with a single-suture closure if the tendon is avulsed or badly crushed, or separate repairs if the tendon is a clean laceration. Management thereafter is similar to that of the closed technique, with a custom thermoplastic orthosis and removal of the K-wire at 6 weeks and a gradual program of intermittent splinting and progressive active flexion. Avulsion fracture of the distal phalanx is reported to cause mallet deformity in as many as 35% of cases and accounted for two thirds of cases with late osteoarthritis 5 years after injury. In as many as 5% of closed mallet injuries, there is an avulsion fracture of the distal phalanx. Two thirds of those who had osteoarthritis 5 years later originally had an associated fracture with a mallet injury.61 However, a decision for surgical versus nonsurgical treatment remains controversial. In general, fractures without DIP subluxation are managed nonsurgically. However, it is debated whether even the subluxated fractures and dislocations are better managed nonoperatively,62 which may be preferable because of the increased complication rates in those managed surgically.40 A study in a cadaver model, involving 29 fingers, showed the DIP joint would subluxate if more than 52% of the joint surface was involved and would not subluxate when less than 40% of the articular surface was involved.63 Others believe that surgical correction and fixation should be performed any time the fracture fragment is greater than one third of the joint surface to avoid subluxation and later posttraumatic osteoarthritis.64 Sometimes, dynamic fluoroscopic imaging of the DIP joint through extension and flexion may be necessary to identify subluxation. When no subluxation is identified, we prefer to manage the injury nonoperatively with a custom thermoplastic splint regardless of fracture size. A variety of surgical treatment options are available, with no proven significant difference in outcomes among any of them65 (Fig. 62.14): • Extension block pinning65–68 • Open reduction internal fixation with pins68,69 • Screws73 • Pullout wires or sutures39,74,75 • Biodegradable arrows76 • “Umbrella handle” technique77 Of all these, extension block pinning is one of the more popular fracture reduction and treatment methods. The distal phalanx is flexed and a K-wire is placed percutaneously through the extensor tendon attached to the bone fracture fragment, thus stabilizing and securing it to the middle phalanx, followed by manual extension reduction of the distal phalanx and longitudinal K-wire fixation across the reduced DIP joint.80 The principles of this technique have also been used by simply using an 18-gauge blunt needle through a small incision, stabilizing the proximal fragment, guiding this reduction under fluoroscopic observation, and then finally using simple extension splinting in hyperextension without K-wires for 6 to 8 weeks.81 This technique of essentially nonoperative intervention may be used to reduce the “bump” commonly seen after healing of non-subluxated but widely separated fractures or dislocations. It might even be used as a first attempt at treatment for subluxated mallet fracture injuries. An especially difficult problem is treatment of a painful late mallet deformity related to chronic nonunited fracture subluxation. These patients present with pain and stiffness. Some suggest proceeding directly to DIP joint fusion. There are reports of successful surgical treatment of these chronically presenting injuries. In a series of 19 patients with more than one-third joint surface involvement who presented on average 57 days after injury, transtendinous wiring was performed with the wire buried against the volar surface (as described by Sonoda et al82), and the fibrous tissue in the fragment gap was débrided to achieve bone contact, together with longitudinal K-wiring and external aluminum orthosis.83 Of these patients, 41% were subluxated at the time of treatment. The average follow-up extension lag was 7 degrees, and flexion ranged from 35 to 95 degrees, but in 21% either bone union failed or the joint became ankylosed. Fig. 62.15 (a) Initial radiograph. Even a chronic painful mallet finger with joint subluxation (3 months after injury) may be reduced (b) by serial casting. (c–e) Bony union occurs with time, resulting in a stable distal interphalangeal joint. (c) After the third week of serial casting and reduction. (d) After 6 weeks of serial casting and reduction. (e) Final reduction and union at 8 weeks of serial casting and reduction. We prefer a nonsurgical attempt that was described in a small series of 10 patients who presented 6 weeks to 6 months after sustaining a mallet fracture. Serial casting was used with progressive manual reduction under fluoroscopic imaging, changing the cast once or twice per week, until the mallet deformity was reduced and then immobilized an additional 8 weeks84 (Fig. 62.15). Eight of ten achieved bone union, and all remained stable. In our experience, this has proven to be a useful and safe technique that is preferable to the morbidity of open reduction and fixation in these late cases. It may, however, leave a dorsal bump where the fracture unites. If this low-morbidity technique fails, the surgeon may still resort to an open treatment. It is surprising that such a common injury as a mallet finger has so much controversy regarding indications for surgery, types of orthoses, the variety of operations, and the length of time for immobilization. An important reason why the variety of treatment options have not shown any significant differences may be because there are no standardized treatment outcome measures and there is also a high variability in the normal range of DIP flexion and natural hyperextension among individuals. Older patients with existing reduced range of motion may, for example, have a poorer outcome by measurement but may actually be pleased with their treatment results. A useful classification of outcomes may be gleaned from the recommendation of Patel et al,52 who compared the treated digit with the individual’s contralateral finger (Table 62.1). The relative-motion concept is addressed here because it is pivotal to management of not only extensor tendon injuries of the dorsum of the hand, but also to sagittal band injuries (zone V) and boutonnière problems (zone III). The only differences are whether one uses the relative-motion extension orthosis or relative-motion flexion orthosis. This concept takes advantage of the “quadriga effect.” Quadriga is a Roman description of a two-wheeled chariot drawn by four horses harnessed abreast and guided by a charioteer with equidistant reins. The Greeks called it a tethripon. Verdan coined the term quadriga effect in 1960 to explain the FDP imbalance that can occur after tendon injury.85 Both the EDC and FDP function as single muscle (“charioteer”) with multiple tendons of fixed lengths (“reins”).86 The quadriga effect or syndrome describes a complication that results in adjacent digits being unable to flex to the palm, either because the repaired tendon is too short and is able to flex because of weakness and lag in the adjacent uninjured profundus tendons, or conversely interphalangeal fusion in too much extension, causing inability of adjacent digits to flex to the palm because of relative loss of flexion to the fused digit.
62
Extensor Tendon Injuries
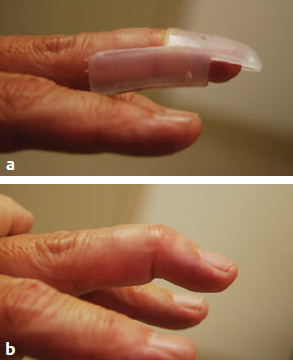
 Dorsal skin pressure necrosis
Dorsal skin pressure necrosis
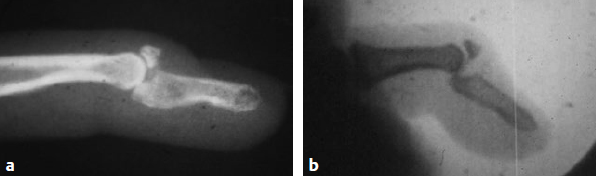
 Inadequate positioning
Inadequate positioning
Avoiding Unfavorable Results and Complications in Extensor Tendon Repair
Biologic Principles
Response of the Entire Organ
Preserve Function in All Structures
One Wound, One Scar
Functional Anatomy of Extensor Tendons
Extrinsic Extensor Tendons
Juncturae Tendinum
Extensor Mechanism at the Metacarpophalangeal and Interphalangeal Levels
Intrinsic Muscles
Stabilizing “Ligaments” of the Extensor Mechanism
Zones of Extensor Tendon Injury
Managing Unfavorable Results and Complications in Extensor Tendon Repair
Mallet Deformity (Zones I and II)
Clinical Assessment and Acute Management
Late and Chronic Mallet Deformity
Surgical Treatment of Mallet Injuries
Treatment of Mallet Fracture
Treatment of a Chronic Subluxated Mallet Fracture
Summary
The Relative-Motion Concept
Plastic Surgery Key
Fastest Plastic Surgery & Dermatology Insight Engine