10 Extensor tendon injuries
Synopsis
 A thorough understanding of the complex anatomy is crucial for successful treatment of extensor tendon injuries.
A thorough understanding of the complex anatomy is crucial for successful treatment of extensor tendon injuries.
 Injuries are classified into nine anatomic zones. Treatment strategies vary considerably according to the location of the lesion, ranging from splinting to tendon grafting.
Injuries are classified into nine anatomic zones. Treatment strategies vary considerably according to the location of the lesion, ranging from splinting to tendon grafting.
 Minimal variations in tendon length may result in considerable alteration in range of motion.
Minimal variations in tendon length may result in considerable alteration in range of motion.
 As in flexor tendon injuries, postoperative care is an essential part of the treatment concept.
As in flexor tendon injuries, postoperative care is an essential part of the treatment concept.
 Closed ruptures of the extensor tendon at the level of the distal interphalangeal (DIP) and proximal interphalangeal (PIP) joints are typically treated conservatively.
Closed ruptures of the extensor tendon at the level of the distal interphalangeal (DIP) and proximal interphalangeal (PIP) joints are typically treated conservatively.
 Lacerations at the level of the metacarpophalangeal (MP) joint (zone V) are not infrequently caused by human bites and are prone to infection unless thoroughly debrided.
Lacerations at the level of the metacarpophalangeal (MP) joint (zone V) are not infrequently caused by human bites and are prone to infection unless thoroughly debrided.
 Ruptures of the sagittal bands may result in subluxation of the extensor tendon at the level of the MP joint.
Ruptures of the sagittal bands may result in subluxation of the extensor tendon at the level of the MP joint.
 The swan-neck deformity is characterized by DIP joint flexion and PIP joint hyperextension. It can be caused by an untreated mallet injury or palmar plate laxity.
The swan-neck deformity is characterized by DIP joint flexion and PIP joint hyperextension. It can be caused by an untreated mallet injury or palmar plate laxity.
 The boutonnière deformity is characterized by hyperextension of the DIP joint and PIP joint flexion. It can be caused by rupture of the central slip of the extensor tendon or palmar subluxation of the lateral bands.
The boutonnière deformity is characterized by hyperextension of the DIP joint and PIP joint flexion. It can be caused by rupture of the central slip of the extensor tendon or palmar subluxation of the lateral bands.
 Complex injuries to the dorsum of the hand can involve skin, tendon, and bone. Adequate debridement is of paramount importance. Before reconstructing tendons, fractures must be stabilized and stable soft-tissue coverage must be provided.
Complex injuries to the dorsum of the hand can involve skin, tendon, and bone. Adequate debridement is of paramount importance. Before reconstructing tendons, fractures must be stabilized and stable soft-tissue coverage must be provided.
Historical perspective
The history of tendon surgery reaches back to about 200 ad. Galen (130–201) mistook the tendons for nerves and suggested in his Ars Parva that no sutures should be placed within the tendons in order not to cause pain and convulsion. The error was not corrected until the 10th century, when Avicenna of Boukhara (980–1037), in Persia, advocated surgical suturing of tendons. However, this new concept did not reach the west until much later – Galen’s dogma was not refuted until the 18th century. The basic structure of the extensor tendon mechanism was illustrated by Albinus in 1734. The next step forward was the concept of tendon transplantation, which was introduced in the late 19th century. In the beginning of the 20th century extensive clinical and experimental research was conducted, especially in Germany. Lexer published the results of 10 flexor tendon grafts in 1912. During the first half of the 20th century, Bunnell developed basic principles of flexor tendon surgery which were published in his masterly book Surgery of the Hand in 1944.1 Fowler and Landsmeer advanced the concept of the balanced forces and dynamics between the flexor and extensor apparatus in the 1940s. During the 1960s operating techniques were introduced that are still in use today.
Basic science/disease process
Anatomy of the extensor tendons
Extrinsic muscles
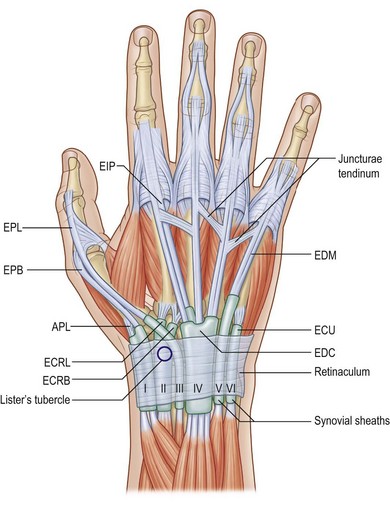
All extrinsic tendons pass through the six compartments of the extensor retinaculum on the back of the wrist (Fig. 10.1). The first compartment is attached to the outer rim of the radius and contains the tendons of the abductor pollicis longus (APL) and extensor pollicis brevis (EPB) muscles. In 34% of patients the compartment is further divided by an additional septum, which has implications for the etiology and treatment of de Quervain’s disease.2 The extensor carpi radialis longus (ECRL) and extensor carpi radialis brevis (ECRB) tendons run through the second compartment, which is bordered by Lister’s tubercle on the ulnar side. The third compartment crosses the wrist in a diagonal fashion above the second compartment, while Lister’s tubercle acts as a pivot point for the extensor pollicis longus (EPL) tendon. While passing through the compartment, the tendon is quite vulnerable to ruptures, e.g., in fractures of the distal radius. The fourth compartment contains both the extensor digitorum communis (EDC) and extensor indicis propius (EIP) tendons. The EDM and extensor carpi ulnaris (ECU) tendons run through the fifth and sixth extensor compartments, respectively. The ECU not only functions as an extensor for the wrist, but is also part of the triangular fibrocartilage complex (TFCC) and thus a major stabilizer for the distal radioulnar joint.
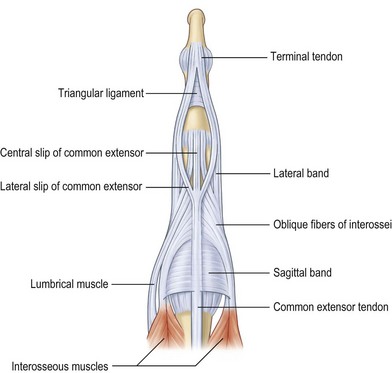
The two extensor proprii tendons of the index and the little finger are located on the ulnar sides of the corresponding communis tendons and allow for individual movements of the peripheral fingers. On the dorsum of the hand, the EDC tendons are interconnected by the juncturae tendinum which facilitate combined extension of the fingers. Lacerations of the extensor tendons which are located proximal to the juncturae may be masked by the function of these bands. The patterns of the intertendinous connnections are highly variably and have been classified into three types: filamentous, fibrous, and tendinous bands.3 At the level of the proximal phalanges, the extensor tendons split up into three parts: the central band and two lateral bands (Fig. 10.2). These merge with the intrinsic extensor system to form the complex extensor apparatus of the digits.
Intrinsic muscles
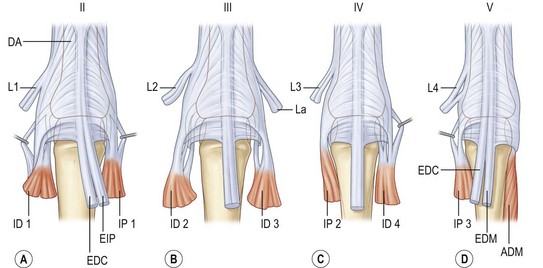
With this arrangement, all four digits have three intrinsic muscles contributing to the extensor apparatus, with the missing ulnar interosseous muscle for the little finger being equivalent to the abductor digiti minimi muscle (Fig. 10.3). The thumb also has three short muscles that join the extensor apparatus: the flexor pollicis brevis (FPB) and abductor pollicis brevis (APB) muscles on the radial side and the adductor pollicis (ADP) muscle on the ulnar side.
Functional anatomy
Linked chains
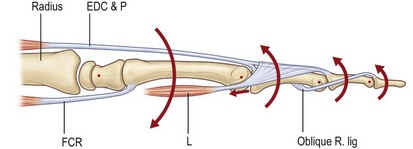
The movement of the fingers is a highly complex mechanism. It is dependent upon a delicate equilibrium between the extrinsic extensor and flexor muscles and the intrinsic muscles. Biomechanically, the finger can be compared to a multiarticular chain comprised of the three phalangeal bones (Fig. 10.4). Landsmeer was able to show that at least three muscles are necessary to control two joints in such a multiarticular chain.4 For the proximal phalanx, these are the extrinsic extensor and flexor muscles and the diagonal intrinsic system (lumbrical and interosseous muscles). In the middle phalanx there is no diagonal muscle system; instead the third component is made up of the oblique retinacular ligament (Landsmeer’s ligament) which has its origin at the flexor pulley and inserts distally into the extensor apparatus. Both of these diagonal systems run palmar to the joint axis proximally and dorsal to the joint axis distally. They play a crucial role in the coordination of extension and flexion movements of the fingers by linking the extrinsic flexor and extensor muscles.
Extrinsic muscle function
It has been shown biomechanically that both the extrinsic flexor and extensor muscles have a component that acts as an extensor on the proximal phalanx. Under physiologic conditions this force is counteracted by the intrinsic muscles. Paralysis of these muscles (as in ulnar nerve palsy) therefore results in hyperextension of the MP joints. Without intrinsic muscle function the long extensors exhaust their potential at the level of the proximal phalanx. Anatomical studies have demonstrated that isolated contraction of the extrinsic extensors results in hyperextended, clawlike position of the MP joints, but not complete finger extension.3 For complete extension of the interphalangeal joints intrinsic muscle function is therefore mandatory.
Mechanisms of joint extension
The MP joint is extended by the extrinsic extensor tendon. However, there have been debates about how tendon force is transmitted to the joint. The variable direct attachment of the tendon to the proximal phalanx has been shown to have no significant contribution to MP joint extension.5 It has been postulated that instead the fibrous connections of the extensor tendon to the flexor sheath are the primary transmitters for extension of the joint.
Extension of the PIP joint is mediated by the central slip of the extensor tendon. However, as stated above, intrinsic muscle function is necessary in order to enable the extrinsic extensor tendon to act on the PIP joint. At the level of the PIP joint, the extensor tendon is centered by the transverse retinacular ligaments. Harris and Rutledge have stressed the importance of the correct position and balance between the central slip and the lateral bands in order to maintain normal PIP extension.5
Until the late 1940s, extension of the DIP joint was thought only to be mediated by the terminal part of the extensor mechanism. In 1949 Landsmeer defined the function of the oblique retinacular ligament which had been unclear since its identification in the 1800s.6 He described the extension of the DIP joint as a combination of the terminal lateral bands and a tenodesis effect mediated by these ligaments. Later these findings were questioned.5 However, dissection of the ligament results in lack of extension of the DIP joint.
Diagnosis/patient presentation
The diagnosis of extensor tendon injuries is often evident. However, partial lesions can be missed if the remaining tendon is strong enough to create some extension force. As a general rule, open lesions should therefore be surgically explored to identify the extent of the injury and prevent secondary ruptures. The function of the EDC tendon should be assessed by extension of the MP joint of the affected digit against resistance. The EPB tendon inserts into the extensor tendon apparatus of the thumb at varying levels and may be able to extend the IP joint of the thumb. If there is a questionable rupture of the EPL tendon, it should therefore not be tested by extension of the IP joint. Instead, the patient should be asked to lift the thumb off the table, which will be impossible without an intact EPL tendon (Fig. 10.5).
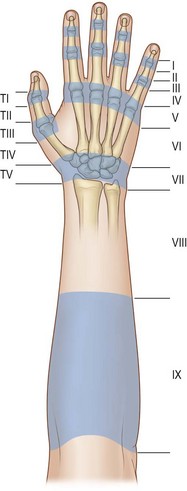
Kleinert and Verdan proposed a system to classify lesions of the extensor tendon apparatus into eight zones according to the level of the lesion.7 Doyle has added a ninth zone by dividing the forearm into the distal (zone 8) and proximal forearm (zone 9).8 This classification is presented in Figure 10.6.
Patient selection
Wide-awake surgery is a concept that is becoming increasingly accepted. In this approach, procedures are performed with no sedation and no tourniquet with the use of tumescent lidocaine and epinephrine. This technique has been proven to be safe and cost-effective.9–12 Most importantly, however, with the patient able to move the fingers during the operation, the surgeon can verify the success of tenolysis or tendon repair procedures immediately.13 Not only tendon repairs and tenolysis, but also tendon transfers can be most effectively performed in this approach (Box 10.1).14
Treatment/surgical technique
Suturing techniques
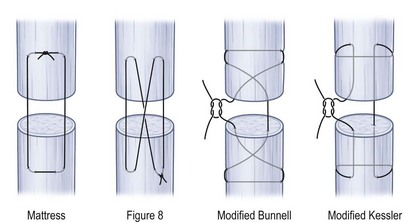
In zones VI and proximally, the extensor tendon resembles a flexor tendon and as such can be repaired with a core suture and an epitendinous running suture. Commonly used suture strengths include 3-0 and 4-0 for core sutures and 5-0 for epitendinous sutures. For neither flexor nor extensor tendons is there any scientific evidence for an advantage of using resorbable or nonresorbable suture materials. Figure 10.7 gives an overview of common types of core suture. In order to achieve maximum core suture strength, locking stitches should be preferred over grasping stitches in order to prevent suture pull-out and reduce gapping.15 However, grasping suture techniques have a higher tensile strength and less gap formation in extensor tendon repair than mattress or figure-of-eight stitches.16 For flexor tendons, it has been shown that at least four core strands should be applied in order to enable early active motion17 and the same is probably true for extensor tendon repair.
In the more distal zones of injury, locking or grasping core stitches become increasingly difficult due to flattening of the tendon. Newport et al. report that grasping stitches in zone IV injuries are strong enough to enable postoperative early active motion.18 Simple running stitches should be avoided due to the low pull-out strength in favor of more complex locking suture techniques (Box 10.2).
Zone I
The mallet finger
Most surgeons prefer conservative treatment with splints over operative therapy for uncomplicated injuries, although the scientific evidence is limited.19,20
Niechajev reviewed 135 patients who had been treated for various types of mallet finger with a minimal follow-up of 12 months.21 The author concludes that operative treatment should only be performed in cases with subluxation of the distal phalanx or avulsed fragments that are more than one-third of the joint surface and with a diastasis of more than 3 mm. Stern and Kastrup reviewed 123 mallet injuries retrospectively.22 Thirty-nine patients were treated surgically, resulting in a complication rate of 53%, including infection, nail deformities, joint incongruities, fixation failure, and bony prominence. The authors conclude that splinting is the preferred treatment option in nearly all mallet fingers. Handoll and Vaghela included four trials in a systematic review.23–27 They concluded that there is insufficient evidence from randomized trials to establish the effectiveness of custom-made or off-the-shelf finger splints, the advantage of surgical treatment over splinting, or even the advantage of splinting over no treatment at all.
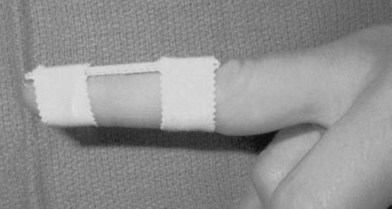
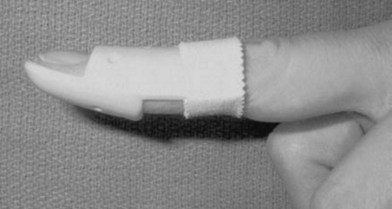
The best available evidence therefore supports conservative treatment by splinting for the majority of cases. Conservative treatment usually implies immobilization of the DIP joint in extension while sparing the PIP joint. By extension or slight hyperextension of the joint the two ruptured ends of the tendon are approximated (Figs 10.8 and 10.9). The fibrous tissue of the resulting scar is thought to be strong enough to restore extension of the joint. The type of splint is not nearly as important as patient compliance. Prefabricated stack splints have been shown to be equally effective as simple aluminum splints or custom sandwich splints (Figs 10.10 and 10.11). Most authors recommend full-time splinting for at least 6–8 weeks followed by a period of 2–6 weeks of splinting at night to enable further shrinking of the immature scar. All patients should be thoroughly instructed to avoid ineffective use of the splints. The splints should only be removed when flexion of the DIP joint by the strong pull of the FDP tendon is counteracted, e.g., through resting the finger flat on a table. By thorough splinting, a residual lack of extension of 10° or less can be expected.28
Surgical treatment for closed injuries should only be considered in fragment sizes greater than one-third of the joint surface. Transfixation of the DIP joint with a Kirschner wire has been suggested for sole treatment of the mallet injury in addition to other surgical interventions.29,30 To avoid scarring of the finger pulp, Tubiana has suggested an oblique angle when crossing the DIP joint.31
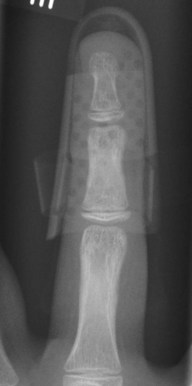
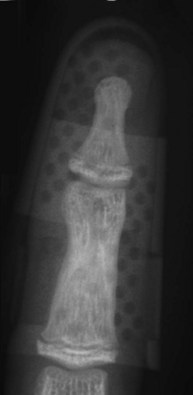
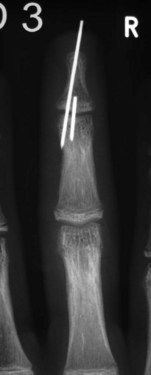
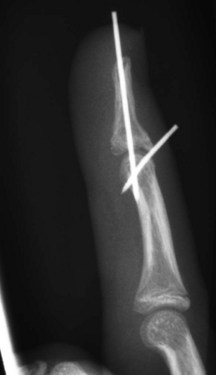
When surgical intervention is indicated, the size of the avulsed fragment should be carefully evaluated for direct fixation. This can be very difficult to achieve and may result in further fragmentation of the avulsed bone. In case of a rather small fragment, indirect reduction by extension block pin fixation should be preferred (“doorstop osteosynthesis”). In this technique the distal phalanx is maximally flexed and a 1.0-mm Kirschner wire is advanced into the middle phalanx dorsal to the avulsed fragment at a 45° angle, creating the extension block against which the fragment is reduced. The joint is then extended, reducing the fragment. This position is secured by a second Kirschner wire inserted longitudinally across the DIP joint for transfixation. The wires are cut and a splint is applied for at least 6 weeks (Figs 10.12–10.15).
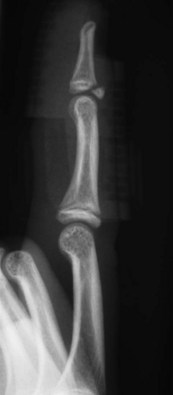
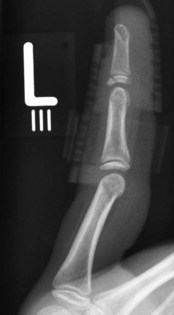
Fig. 10.13 Mallet injury: lateral view. Despite the stack splint, the fragment is not properly reduced.
If the avulsed fragment seems large enough, it may either be pinned directly percutaneously or reduced in an open fashion through a zigzag incision of the distal and middle phalanges. In case of open reduction, screw fixation seems preferable. Alternatively, a pull-out suture may be applied, as described by Doyle.8
Zone II
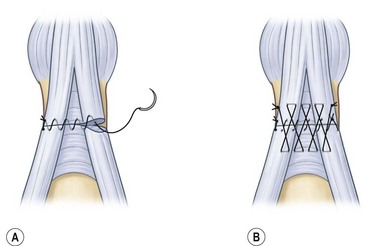
Injuries to the extensor tendon over the middle phalanx usually result from sharp, direct lacerations or crush injuries. Acute lacerations should be explored to determine the extent of the tendon injury. If less than 50% of the tendon substance are injured, the tendon is considered stable and no further treatment is necessary. If more than half of the tendon is involved, additional suturing is necessary. When evaluating these injuries, phalangeal extension should always be tested against resistance. Doyle recommended a running stitch combined with a Silfverskiöld cross-stitch8 (Fig. 10.16). Care should be taken to avoid considerable shortening of the tendon which will result in lack of flexion of the DIP joint.