14 Expander-implants breast reconstructions
Synopsis
 Implant-based breast reconstructions can be employed in all patients, provided that they have not been previously irradiated.
Implant-based breast reconstructions can be employed in all patients, provided that they have not been previously irradiated.
 Medium size anatomically-shaped permanent silicone implants can be employed to reconstruct virtually all breasts, irrespective of shape and size.
Medium size anatomically-shaped permanent silicone implants can be employed to reconstruct virtually all breasts, irrespective of shape and size.
 A contralateral adjustment should be part of the reconstructive project.
A contralateral adjustment should be part of the reconstructive project.
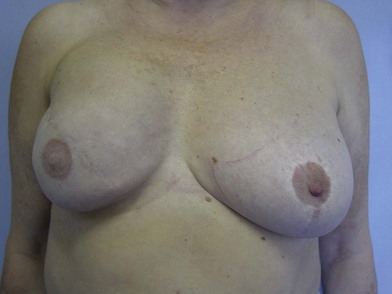
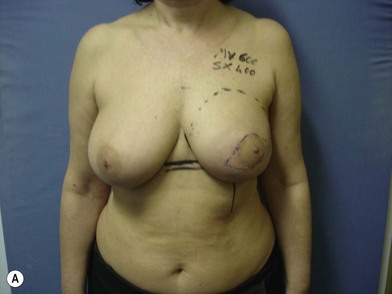
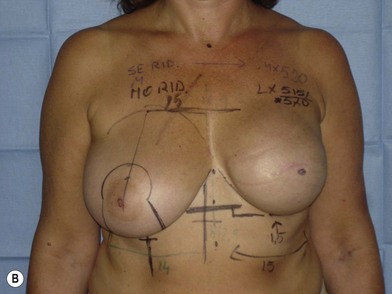
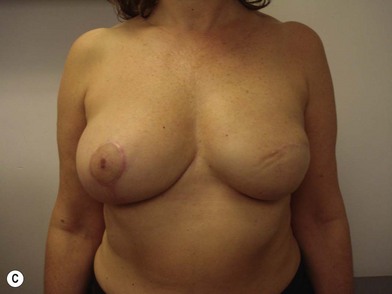
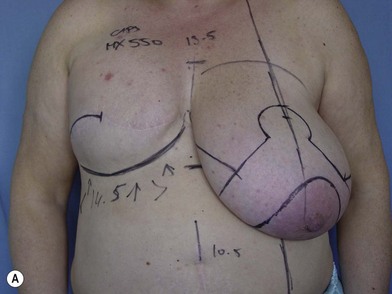
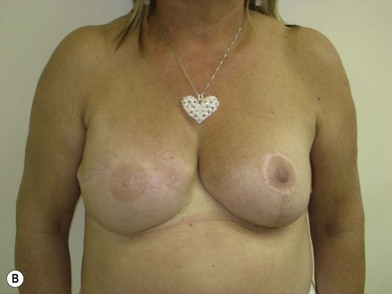
 Breast reconstruction in the large breast can be accomplished in one stage (“skin reducing mastectomy”).
Breast reconstruction in the large breast can be accomplished in one stage (“skin reducing mastectomy”).
History
Development of breast implants
The history of breast implants starts in the 19th century, when the first attempts to augment a breast were recorded. Ironically, and in-keeping with modern research on tissue regeneration, one of the first techniques described was based on the transfer of a lipoma from the back to the breast area.1 Further attempts were made across the years, adding the most heterogeneous materials (from cartilage to soya-bean oil, including a various number of copolymers). Free silicone was used for augmentation in a large number of women who developed severe complications because of this practice. Silicone implants, as currently engineered, were employed for the first time at the beginning of the 1960s. A silicone bag filled with fluid silicone was inserted for breast augmentation. The first generation of implants had a quite thick shell to prevent gel migration; some products were provided with anchoring systems that demonstrated their uselessness and created stress points in the prosthesis, with possible ruptures and gel migration. The inner gel was quite viscous to keep a proper shape.
Basic science
Basic science in the field of breast implants, no differently from other branches of plastic surgery, is affected by a generalized lack of evidence. Most of the researches currently published in international journals are based on very empirical observations and rely mainly on expert opinions or on small retrospective series. A recent systematic review on the assessment of health-related quality of life included2 a total of 34 papers and most of them appeared to be compromised by poor statistics, lack of reproducibility due to the use of generic instruments of evaluation, reliance on single center’s observations and no unequivocal reports on complications. Furthermore, among the 34 examined papers, only two were based on level 1 evidence and 11 on level 2. The authors conclude that there is an urgent need for standardized instruments for outcome evaluation to provide a reliable guidance for further research.
Basic research on implants is strictly tightened to breast shape assessment. A quantitative analysis of breast shape and of its modifications over times is not possible, the few tools proposed in literature have been tested by our group with very disappointing results. A more precise geometrical language should be available and estimates from geometry of curved surfaces should replace currents linear measurements that are unable to assess complex round territories like the female breast.3
Research efforts should also be made to analyze the breast deformation during walking or with the movements of the trunk and of the arms. In fact, the breast is not a static object and capsular contracture mainly affects breast softness and plasticity.4,5
Capsular contracture has been widely investigated as the major trade-off of prosthetic reconstructions. Several explanations have been proposed according to endogenous or exogenous hypothesis and capsular contracture appears to be a multifactorial clinical condition related to surgical technique, implant manufacturing, anatomical plane of implantation, etc. Infections can be the leading cause generating this condition.6,7 This theory gives explanations for asymmetric contractures and for effective reduction using implants in association with topic antibiotics or iodopovidone,7 and is supported by studies that demonstrate a much higher percentage of positive culture rates in severely contracted capsules (89.5% vs 10.5% in grades I and II).8
Any convincing theory for capsular contracture is tightly related to an upregulated response to foreign bodies. Several logistic mediators, together with fibroblasts, macrophages and CD4 are involved in this process, and it has been argued that a good treatment could be related to topic or systemic administration of anti-inflammatory drugs. Leukotrienes such as zafirlukast have been investigated with positive results.9
Our research group confirmed this observation in an experimental study,10 in which we evaluated the effectiveness of zafirlukast. Disks of textured implant material were placed dorsally into each of the subcutaneous tissues of 40 rats that were subdivided into two groups: 20 rats treated with zafirlukast and 20 controls. At autopsy 77 days after treatment, each implant with its surrounding collagenic tissue was excised, and the macroscopic measure of the membrane thickness was compared with the pathology reports, to definitely assess the foreign body reaction. The mean total thickness of the capsule around the implants was 161.97 µm in the zafirlukast-treated group compared with 345.98 µm in the control group (p<0.001). Outstandingly, the collagen fibres and fibroblast layer were reduced in the zafirlukast-treated group compared with the controls. Our study confirms the effectiveness of this compound in preventing fibrosis and reducing the extent of collagen reaction when a capsule has been formed.
Several clinical investigations of the effects of shell texturization in preventing capsular contracture were conducted. Barnsley11 performed a meta-analysis of seven trials12–20 comparing smooth and textured implants; only three of these studies demonstrated significantly lower rates with the use of textured implants. However, a pooled analysis of all seven studies demonstrated an odds ratio (OR) of 0.19 indicating a protective effect for surface texturing on the rate of capsular contracture. The only subgroup who did not benefit from texturization was the one belonging to a single trial, in which the implants were put in a sub-muscular position.
A further meta-analysis of trials of subglandular breast augmentation by Chin-Ho Wong21 reported similar results, although several limitations were correctly pointed out by the authors. Although in the presence of clinical trials with a robust design (prospective controlled randomized), the outcome evaluation is substantially biased by subjective and non-reproducible observations, as capsular contracture was assessed according to the subjective Baker scale. The authors correctly stated that clinically important contractures (Baker III and IV) were defined as capsular contracture in all studies and that two or more observer independent examiners were employed for patient evaluation and that discordant opinions were solved by consensus. Moreover, other limitations arise from the lack of standardization of surgical techniques and incision approaches, the short-term follow-up (only two studies with a follow-up longer than 1 year), with several patients lost to follow-up and consequently data deterioration after the first year of observation. Notwithstanding the efforts in providing a higher level of evidence, more objective and quantitative tools for assessment of the outcome of plastic surgery of the breast should be provided.
The use of human acellular dermal matrix has been reported as a possible tool for prevention of capsular contracture. A study by Basu and colleagues22 on 20 patients who underwent two stage breast reconstruction, investigated the histopathologic characteristics of the capsule in the bio-integrated area and in the native subpectoral capsule. A semi-quantitative analysis was performed, the scores were statistically analyzed and significant differences were observed in favor of the acellular dermis. The acellular dermal matrix facilitates breast reconstruction, avoiding the harvesting of the serratus muscle; it also provides a good definition to the lower pole of the breast and a more natural ptosis. However, in this study it is demonstrated that the acellular dermal sheet, consisting in a biological matrix deprived of cells, allows the implantation of cells and vessels from the host, establishing a physiological matrix cell interaction. For this reason, host cells are able to revitalize the exogenous matrix, also containing extracellular matrix proteins (such as hyaluronic acid, fibronectin, collagen, and fibronectin), facilitating normal wound healing and avoiding chronic inflammatory changes and foreign body giant cell formation.23–25
The largest series on this device reports on two groups of patients who underwent breast reconstruction either using the acellular device or not. The seroma and infection rates were higher in the acellular dermal matrix group (14.1% vs 2.7%, p = 0.0003, for seroma; 8.9% vs 2.1%, p = 0.0328, for infection) and acellular dermal matrix and body mass index were statistically significant risk factors for developing seroma and infection. The authors concluded that although the acellular dermal matrix enhances final results, a higher short-term complication rate is awaited (OR seroma 4.24 times, p = 0.018 and infection 5.37 times, p = 0.006).26
Similar conclusions are report by Newman et al.27 in a recent meta-analysis, in which they concluded that the incidence of short-term complications with human acellular matrices is approximately 12%.
Breast reconstruction with tissue expansion and permanent implants
Although Neumann’s28 report appeared in Plastic and Reconstructive Surgery and demonstrated the feasibility of the procedure, major interest in tissue expansion did not occur for another 20 years. Working independently, Radovan29 and Austad30 developed silicone tissue expanders and published their findings in 1982. Radovan performed his first tissue expansion in 1976. Austad developed a self-inflating silicone prosthesis and investigated the histologic effects of tissue expansion. Subsequent to this early work, tissue expansion has been investigated thoroughly and gained widespread acceptance on the basis of its proven safety and efficacy.
The advantages of the expander-implant technique for breast reconstruction include the following:
• Reduced operative time. Although there are usually two procedures, each is short in the range of 1–1.5 h and requires only one night in hospital. The second stage may be performed on an outpatient basis according to the preference of the patient and the surgeon
• No donor site morbidity, unlike in flap procedures, when the patient will have an additional scar at the flap harvest site
• If the patient becomes dissatisfied with the result, all pre-existing flaps are still available, and the expander-implant maintains the breast space if the flap is later incorporated into a secondary reconstruction
The disadvantages related to expander-implant use include the following:
• Complications inherent to implant use, including implant deflation or malfunction, capsular contracture, and fear of adverse interactions between the patient’s immune system and the device
• Contour irregularities visible on skin surface due to underlying implant. Again, because the implant is gradually encapsulated with scar, the adhesions of the scar to the implant and skin may result in unnatural appearance
• The implant will not behave like normal vascularized tissue. It will remain cooler than adjacent body parts when ambient temperature is low, and the reconstructed breast will not develop natural ptosis with advancing age because of scar attachment between implant and chest wall and overlying skin envelope as opposed to the contralateral breast
• The patient must have an adequate skin envelope to support the expander-implant. In delayed reconstruction, irradiated skin represents a relative contraindication to the expander-implant because implant exposure may occur and the skin envelope will usually not respond to the expansion process. If the patient is a smoker or is being treated for scleroderma, use of an expander-implant is a relative contraindication.
• Cessation of smoking for 6 weeks may be acceptable to proceed with the expander-implant, although skin circulation may still be adversely affected. The patient must agree to delayed surgery of the opposite breast to establish symmetry with the reconstructed breast mound (reduction mammaplasty or mastopexy, augmentation).
The patient must be well informed about all options for breast reconstruction. In general, autogenous breast reconstruction will provide a more natural breast but will require more complex surgery and additional donor site scars. The patient must be willing to accept the use of a permanent prosthesis. At present, both the silicone gel- and saline-filled implants are approved by the Food and Drug Administration (FDA) for breast reconstruction.31
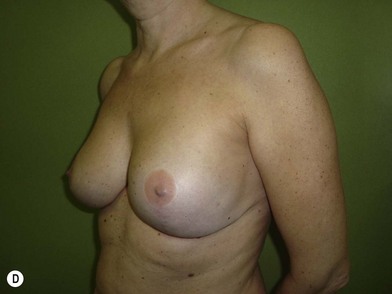
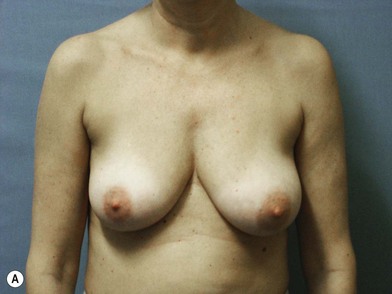
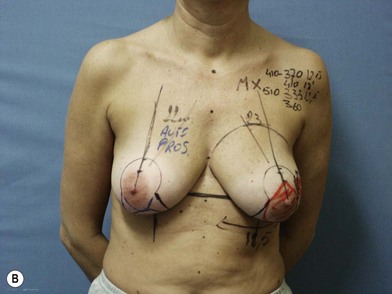
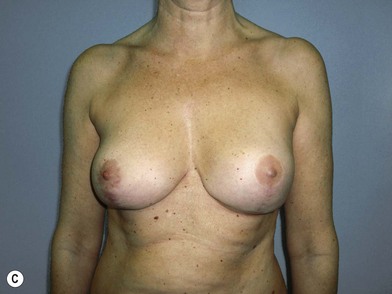
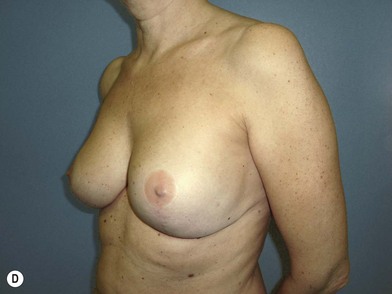
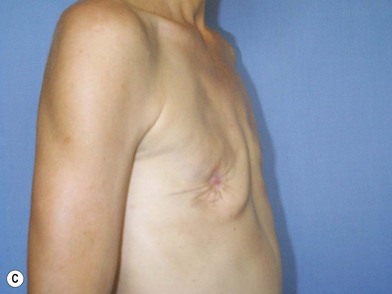
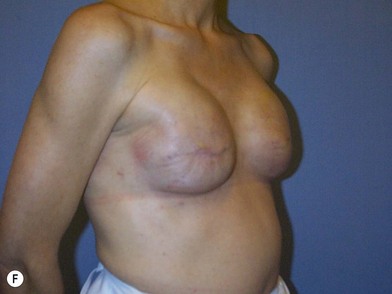
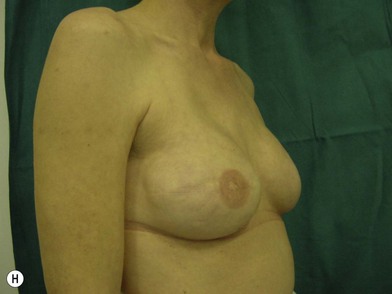
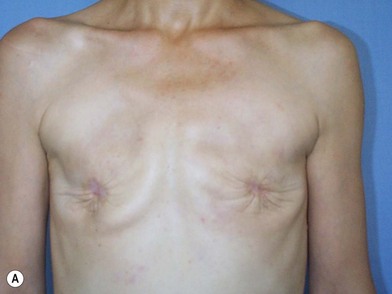
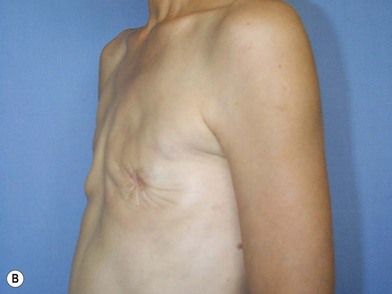
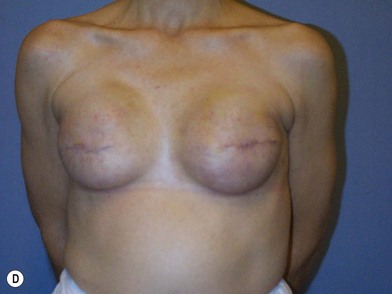
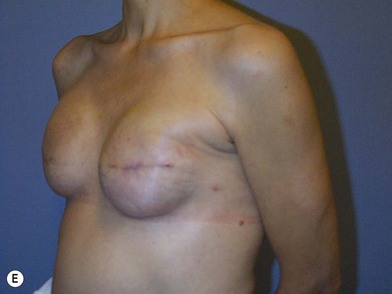
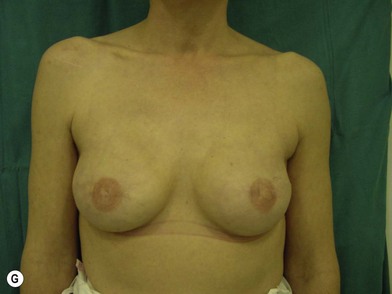
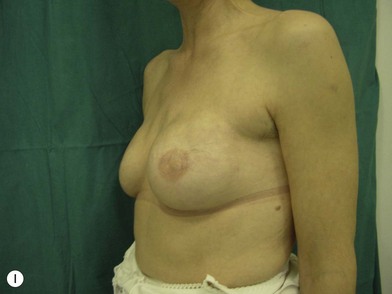
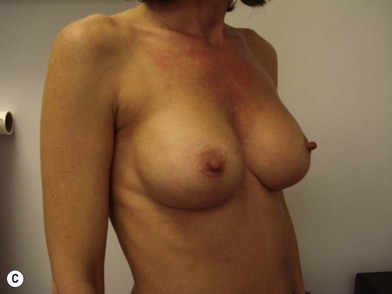
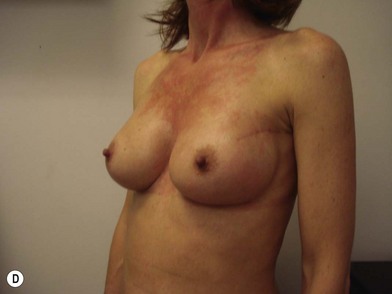
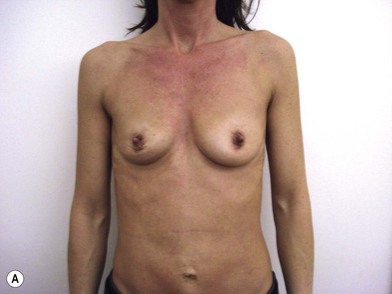
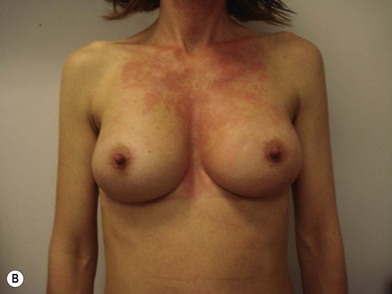
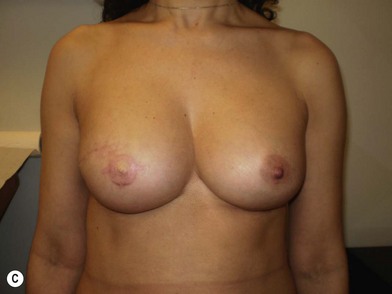
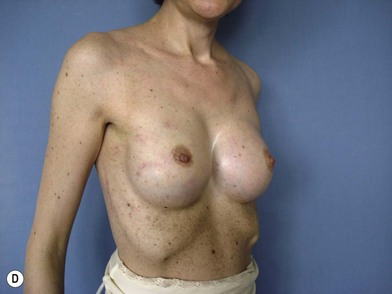
The expander-implant breast reconstructions have evolved during the last few years (Fig. 14.1). In the past, prostheses were mainly employed to reconstruct small breasts; nowadays, thanks to the modern anatomical devices, it possible to reconstruct a cosmetic medium-sized bosom (with contralateral adjustment) for all patients, independently from the original breast shape. We contraindicate this technique only to previously radio-treated patients.
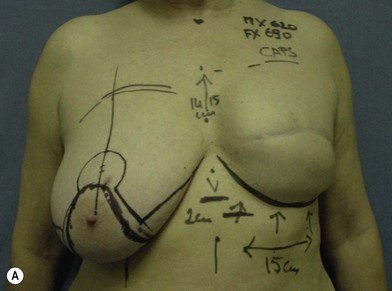
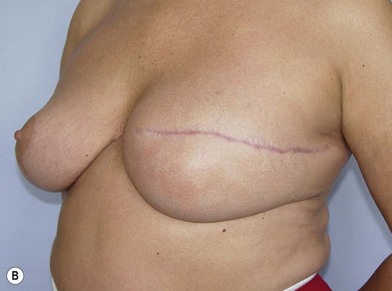
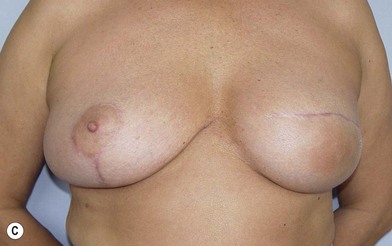
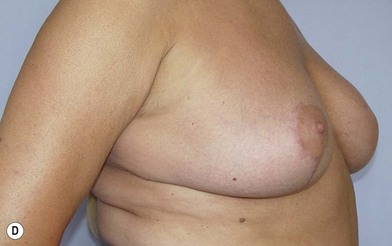
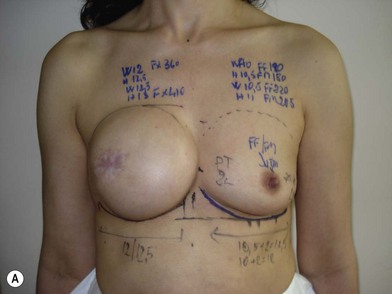
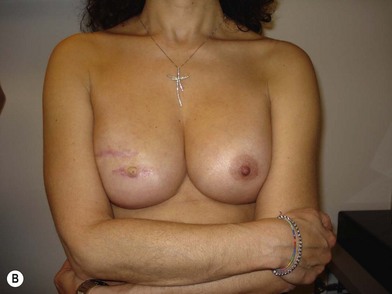
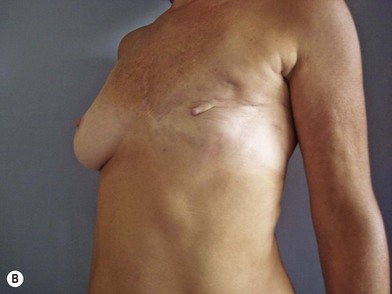
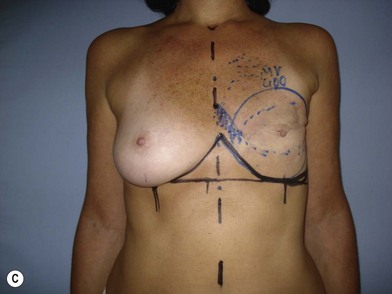
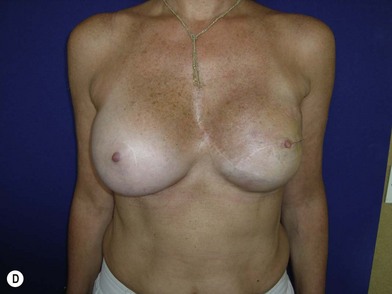
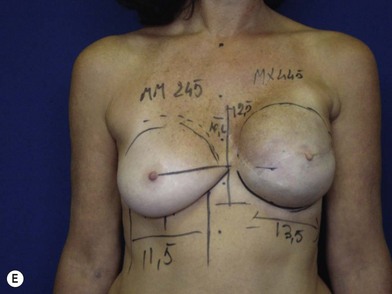
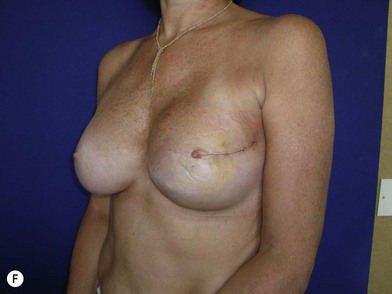
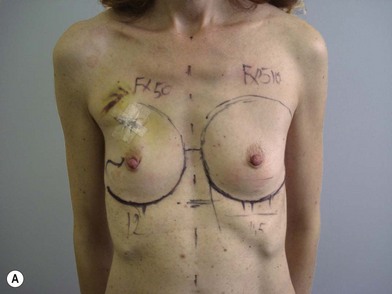
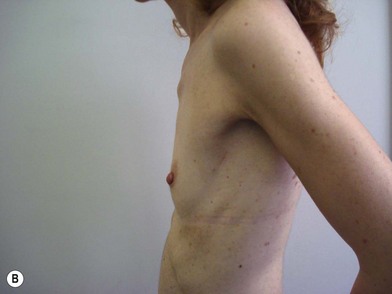
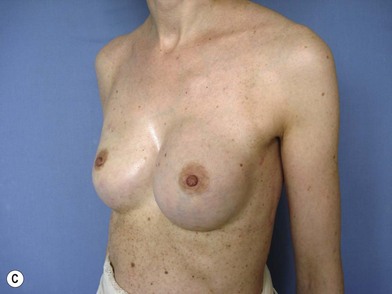
We validated a modern surgical model for two stage reconstructions and we expanded the indication to one stage reconstruction with a new technique called “Skin reducing mastectomy” (Fig. 14.2). This technique is currently under development at our institution and it allows one stage reconstruction in women with large breasts with contralateral symmetrical scarring. Implant reconstruction can be performed either in the same surgical time of the mastectomy or as a delayed procedure (Fig. 14.3).
Reconstructive paradigms and surgical strategy
An established paradigm for breast reconstructions to rebuild, after mastectomy, an identical and possibly symmetrical breast mound is required. Autologous flaps were favored in the reconstruction of large and ptotic breasts due to their ability to reproduce a natural symmetry even with a contralateral ptotic gland. Reconstructions with sub-pectoral implants were indicated mainly for small and medium-sized glands with a moderate degree of ptosis. Operations on the healthy breast in search of symmetry were considered undesirable, as they were expected to compromise surveillance on contralateral disease.32,33
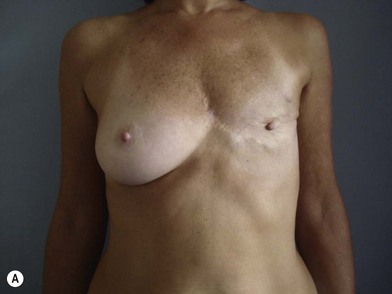
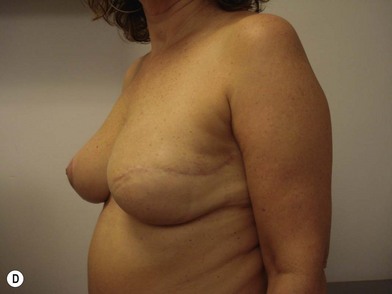
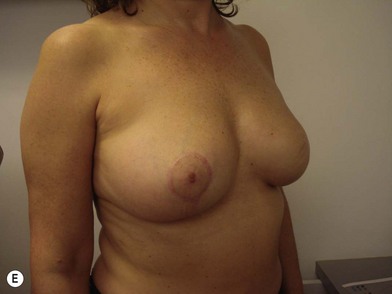
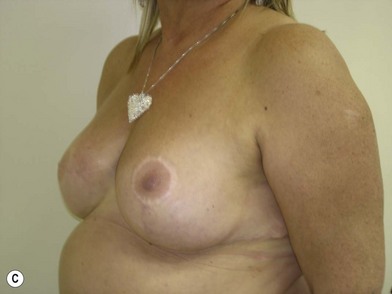
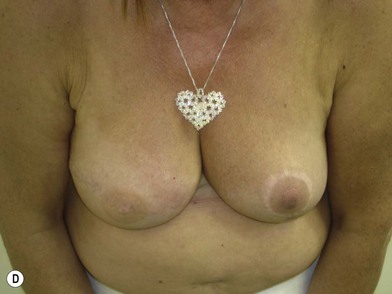
Extra-projection devices gave us the chance to modify this reconstructive predicament (Figs 14.4, 14.5). Modern anatomically-shaped implants can be employed for breast reconstruction and can spare women from complex operations that, as far as myocutaneous flaps are concerned, can generate severe biomechanical complications.
Fig. 14.4 Indications and contraindications in implant based breast reconstructions in radio-treated patients.
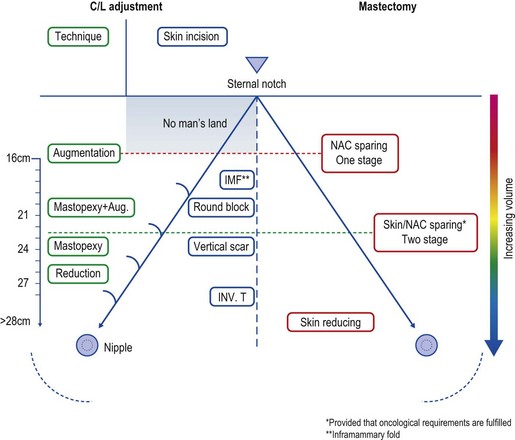
Fig. 14.5 Mastectomy techniques and contralateral adjustment according to breast measurements and morphology.
Extra-projection prostheses filled with highly cohesive gel can yield not only a rewarding cosmetic outcome but a safe surgical approach (Fig. 14.6).34,35
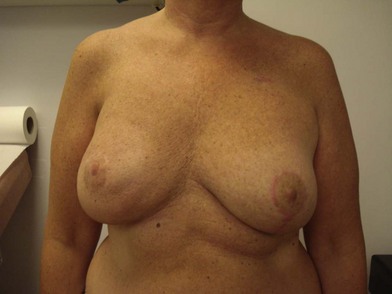
In the authors’ experience, breast reconstructive surgery aims to create, for all women, a bilateral cosmetic medium-sized breast (400–500 cc), highly projected, with little to moderate ptosis, rather than a ptotic gland exactly matching the contralateral. This is demonstrated by the medium volume of implanted prosthesis in comparison to the contralateral adjustment technique; this ranges from 397 cc for women with small breasts who received an augmentation, to 533 cc for those whose healthy side required reduction surgery.36
Our approach substantially differs from that reported by Losken et al.33 in one of the largest series on breast reconstructions. In this work, one-third of patients who underwent breast reconstruction did not receive contralateral adjustment, whereas this incidence in our experience does not exceed one case out of ten.
The observation of sub-groups stratified according to operations on the opposite site demonstrated that the cosmetic and reconstructive purpose of this methodology is emphasized when implants are used also contralaterally (Fig. 14.7). A higher satisfaction rate is reported in this case, while on the other hand ptotic breasts treated only by mastopexy tend to recur over years (Fig. 14.8). We are aware that women who have developed breast cancer are at a higher risk of a second malignancy in the contralateral breast;37 for this reason contralateral augmentations in this setting are still debatable.
In healthy women, it is well known that despite the diminished sensitivity of mammography with implants, augmented and nonaugmented patients are diagnosed at a similar stage and have a comparable prognosis.36 Much longer follow-up in this study and the increased role of MRI will probably also clarify this aspect.38
Diagnosis and patient presentation
Exclusion criteria
Prosthesis for reconstructions can be employed in all women undergoing immediate or delayed breast reconstructions that did not receive previous radiation. Several studies demonstrated a higher complication rate after implant positioning in a radio-treated field.39–41 Similar observations are reported for the reconstructed breast receiving radiotherapy on temporary expanders. For this reason, patients preoperatively scheduled to undergo radiotherapy for locally advanced breast cancer are discouraged to undergo an immediate reconstruction and a delayed flap-based reconstruction is recommended (Fig. 14.9).
Radiation therapy determinates a progressive change of the skin surface that originates in an inflammatory chronic condition. We can observe early effects and long-term effects. The first occur within 90 days of treatment and include dryness, epilation, pigmentation changes, and erythema.42 After 90 days, a chronic logistic condition may appear with progressive induration of the skin, fibrosis, and oedema. The ultrastructural analysis of this condition reveals clear signs of ischemia, with capillary vessels reduced in number and exhibiting duplication of the basal membrane, ectatic lumen cytoplasmic activation of endothelial cells.43 Any attempt to perform alloplastic reconstruction in this setting of chronic ischemia has demonstrated to yield an unacceptable rate of severe complications with implant extrusion, capsular contracture or implant displacement.39,44 A trial is ongoing in our institution for patients whose indication for radiation is not known before mastectomy. In such cases we perform an immediate two-stage breast reconstruction. Patients undergo tissue expansion during postoperative chemotherapy and once the second stage of the reconstruction has been accomplished, they receive radiation on the permanent implant. According to preliminary results, this strategy has extended the indications for implant-based reconstruction also to women requiring post-mastectomy radiotherapy. This strategy allows reduction of the complication and extrusion rates, especially in comparison with patients who receive radiation on tissue expanders. The capsular contracture rate of irradiated breast reconstruction is clearly higher and in accordance with that reported by other authors.45 However, we observed satisfaction rates not particularly different from that reported by patients who did not received radiation.
Fat grafting to treat radio-induced damages of soft tissue is being widely employed in the field of breast reconstruction with prosthesis (Fig. 14.10). This simple procedure, commonly performed under local anesthesia, has changed the fate of reconstructions at high risk of complications. Since the first report from Rigotti et al.,43 who described the effectiveness of transplantation of lipo-aspirates to treat radio-induced inflammation, other authors have confirmed the effectiveness of this technique. Serra-Renom et al.46 for instance, recently demonstrated that in mastectomized patients who received radiotherapy, fat grafting in addition to traditional tissue expander and implant breast reconstruction will lead to better reconstructive outcomes with the creation of new subcutaneous tissue, accompanied by improved skin quality of the reconstructed breast without capsular contracture.
Inclusion criteria according to dimensional considerations
Patients with small breasts
These patients can be candidates for immediate one stage reconstructions. If a delayed reconstruction is required, this will certainly require two stages (Fig. 14.11).
Patients with medium-sizes breasts
This is the largest subgroup of patients in our experience; a two-stage immediate or delayed expander-implant procedure provides the best results (Fig. 14.12).