Excision Followed by Radiofrequency Ablation (eRFA)
V. Suzanne Klimberg
Cristiano Boneti
Introduction
Lumpectomy followed by radiation is widely utilized for the treatment of breast cancer. At least 75% to 90% of recurrences occur at the previous lumpectomy site and positive margins are found in 20% to 55% of lumpectomy specimens. Radiation effectively lowers the risk at the site of the lumpectomy but is not as important in reducing ipsilateral elsewhere recurrences in the breast—giving rise to the concept and the effective treatment of brachytherapy that treats approximately a 1-cm area around the tumor bed with 100% radiation dose. Excision followed by radiofrequency ablation (eRFA), in a similar way, utilizes the radiofrequency ablation (RFA) to in effect extend the margins of the lumpectomy by 1 cm and ablate any undetectable residual disease. This procedure is designed to increase the margin in primary lumpectomy cavities without further excision, thereby avoiding additional tissue resection and maintaining optimum cosmesis in breast-conserving surgery (Fig. 15.1).
Significant preclinical and clinical data have demonstrated that RFA applied to a lumpectomy cavity can ablate a consistent centimeter around the cavity heating to 100°F for 15 minutes. This concept has been demonstrated using whole mount reconstruction of simulated lumpectomies in mastectomy specimens treated with eRFA. A multi-institutional trial in Italy showed that in vivo eRFA of invasive breast cancers followed by standard quadrantectomy showed at least a 1-cm circumferential ablation around the lumpectomy sites. It has also been shown effective in a pilot clinical trial in the United States to evaluate eRFA, 41 patients underwent lumpectomy followed by intraoperative RFA. Eleven of 41 patients had inadequate margins; however, only 1 required re-excision for a grossly positive margin. Biopsy of the cavity walls postablation demonstrated at least a 1-cm thick ablation. Seventeen of the 41 patients had postoperative radiation therapy. During the median follow-up of 24 months, there were no local recurrences. Two of the patients have had recurrence distant to the operative site. Recently, an update of this trial with 94 patients with nearly 2-year follow-up showed no in-site recurrences. A multi-institutional trial in the United States will further evaluate
this procedure. In addition, Klimberg and colleagues have recently developed a method for following the in vivo ablation with Doppler. Because of the off-gassing of nitrogen, the zone of ablation as well as its proximity to the skin can be measured via the color Doppler. This allows not only assurance of a 1-cm thickness of ablation but a real-time way to avoid skin burns.
this procedure. In addition, Klimberg and colleagues have recently developed a method for following the in vivo ablation with Doppler. Because of the off-gassing of nitrogen, the zone of ablation as well as its proximity to the skin can be measured via the color Doppler. This allows not only assurance of a 1-cm thickness of ablation but a real-time way to avoid skin burns.
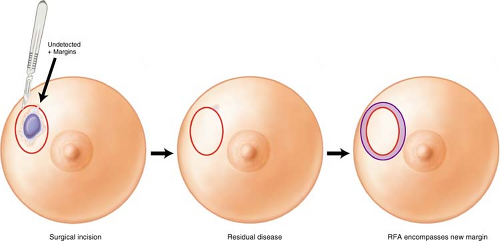 Figure 15.1 Concept of excision followed by radiofrequency ablation to extend the margins of the lumpectomy site. |
The indications for eRFA—RFA after a lumpectomy for cancer (invasive or noninvasive cancer) include the following:
Extend margin width without resecting additional tissue and by doing so decrease the possibility of re-excision for close margins.
Ablate occult and otherwise undetectable disease in patients who cannot be treated with radiation therapy (XRT) (e.g., collagen vascular disease, comorbid diseases, prior XRT for conservative breast cancer treatment) or who refuse XRT or where XRT is of questionable value.
Contraindications include the following:
Patients with pacemakers (unknown risk)
Patients with skin thickness after excision of less than 1.0 cm
Order of Procedures
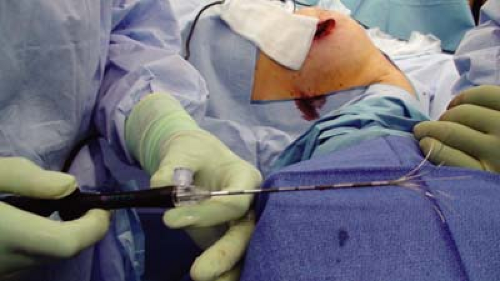
The radiopharmaceutical and/or blue dye is injected into the breast first or blue dye is injected into the arm for axillary reverse mapping (ARM). The sentinel lymph node biopsy is then performed (see Chapter 10) first. The excision or lumpectomy is then performed (see Chapter 14). Then, the excision bed can be prepared for eRFA (Fig. 15.2).
Radiofrequency Probe
The RITA StarBurst XL (AngioDynamics, Inc., Latham, NY) is the device used for all the procedures to date, but it should be possible to perform eRFA with any device that is able to monitor temperature. The tines of the array can be deployed up to 7 cm in diameter (Fig. 15.3). They are easily retracted for repositioning. The probe has four thermal tines spaced in between five tines for monitoring temperature. The sterile RFA probe is placed on the field separately. A sterile reusable connector cable is also placed on the field.
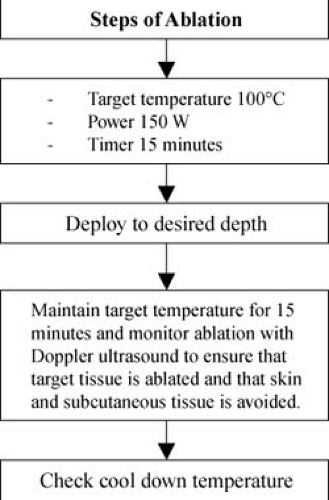
Generator Settings
The RFA generator is set to a power of 150 W, a temperature of 100°C—average of all and 15 minutes of ablation (three windows on left of generator) (Fig. 15.4). Five windows (to right within the circle) interactively indicate the five temperature tines.
Placement of Grounding Pads
Two grounding pads are placed for the eRFA and are placed away from the electrocautery grounding pads. They should also be placed away from any metal prosthesis—hip or otherwise.
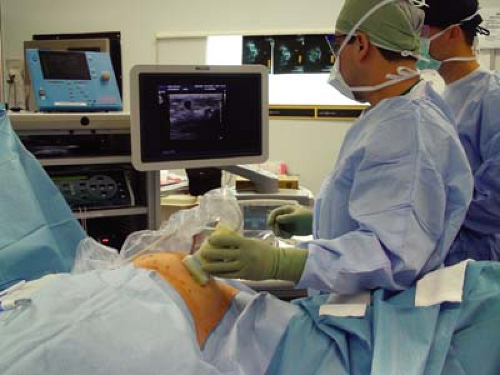
Ultrasound/Doppler
Any standard ultrasound (US) with a Doppler mode can be used. A 7.5- to 10-MHz probe usually gives the appropriate depth to be monitored.
Relevant Anatomy
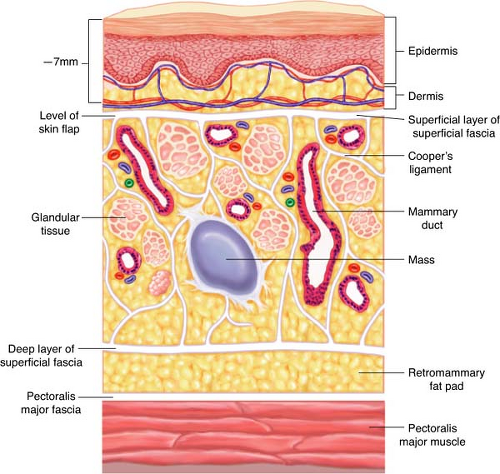
Figure 15.5 demonstrates the relevant anatomy of the overlying breast. The cosmetic result of any lumpectomy depends on the depth of the subcutaneous tissue underneath the incision. Thus, if only thin flaps will remain after the resection of a superficial tumor, it is best to resect an overlying ellipse of skin. If the tumor is deep and muscle is ablated, it is important to free that zone circumferentially in the plane of the retromammary fat pad so that the ablation is not fixed to the chest wall.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree