Chapter 10 Evaluation of the burn wound
Management decisions
![]() Access the complete reference list online at http://www.expertconsult.com
Access the complete reference list online at http://www.expertconsult.com
![]() IN THIS CHAPTER
IN THIS CHAPTER ![]() PowerPoint Presentation Online
PowerPoint Presentation Online
Introduction
Perhaps the greatest advance in burn care to date has been the institution of early surgical excision of the burn wound with immediate or delayed wound closure strategy individualized to each patient.1–4 For many years, burns were treated by daily washing, removal of loose dead tissue, and application of some sort of topical nostrum until wounds healed by themselves or granulation tissue appeared in the wound bed. Superficial dermal burns healed within 2 weeks and deep dermal burns healed over many weeks if infection was prevented. Full-thickness burns lost their eschar in 2–6 weeks by enzyme production from bacteria and by daily bedside debridement. Split-thickness skin grafts were applied usually 3–8 weeks after injury. A 50% graft survival was considered acceptable and repeated grafting eventually closed the wound. The prolonged and intense inflammatory response made hypertrophic scars and contractures part of normal burn treatment.
Pathophysiology of the burn wound
Skin biology
The skin is the largest organ in the human body and is comprised of two layers: the epidermis and the dermis. The thickness of the epidermis varies among different parts of the body, from 0.05 mm on the eyelids to over 1 mm on the soles.5 Most of the skin thickness comes from the dermis, which varies with age, gender, and body location. The skin serves as protection against fluid and electrolyte loss, infection, radiation, and provides thermal regulation. Contact with the skin provides the individual clues to the surrounding environment through touch, perception of temperature and pain. In addition, skin appearance is a major determinant of identity and affects interpersonal interactions.
The mostly cellular epidermis is derived from ectoderm and the principal cell is the keratinocyte. These cells begin their division and differentiation at the basal layer and move progressively outward over 2–4 weeks6 along the outer four layers of the epidermis: the stratum spinosum, the stratum granulosum, the stratum lucidum, and the stratum corneum. Keratinocytes lose their nuclei in the stratum lucidum and become flattened dead cells in the stratum corneum. Other important cells of the epidermis include melanocytes, which produce the melanin pigment essential for protection against ultraviolet radiation, and Langerhans cells, which perform phagocytosis and presentation of foreign antigens. The epidermis, because it is derived from ectoderm, is capable of regenerative healing. Thus, pure epidermal injuries heal by regeneration and without scarring. Keratinocytes proliferate from dermal appendages (hair follicles, sweat glands) and the edges of the wound to achieve reepithelialization. Depleted melanocytes after injury, however, regenerate more slowly and less predictably, which may lead to permanent pigment changes within the healed wound.7,8
The basement membrane zone connects the epidermis to the dermis via epidermal projections (rete ridges) that interdigitate with dermal projections (papillae). The critical structures that stabilize the epidermal-dermal junction are keratinocyte-derived collagen VII anchoring fibrils that extend into the dermis.9,10 These anchoring fibrils may take several weeks (and sometimes months) to mature during burn wound healing. Minor shearing forces may cause shearing, blistering and sometimes epidermal loss until the interdigitations mature.
The dermis with its abundant extracellular matrix component is derived from mesoderm and is divided into the more superficial papillary dermis and the deeper reticular dermis. Collagen fibers provide the bulk of the dermal structure. Their organized orientation allows for stretching and tensile strength of the skin.11 Elastic fibers impart the elastic recoil properties of skin. Protein turnover (by degradation, production and remodeling) increases with mechanical stress and during healing, accounting for the high plasticity of skin. Collagen and elastin are both synthesized by fibroblasts, the principal cell of the dermis. The non-fibrous component of the dermis is called the ground substance. It is composed of glycosaminoglycans and proteoglycans such as hyaluronic acid and chondroitin sulfate, whose function is to entrap fluid to maintain the semi-fluid matrix and to regulate cellular cross-talk by binding and releasing inflammatory mediators.12 Adnexal structures (sweat glands, sebaceous glands, and hair follicles) originate in the dermis and extend through the epidermis. Since they are lined with epidermal keratinocytes, adnexal structures provide the epithelial cells necessary for reepithelialization after a partial dermal injury. The dermal plexus of capillary vessels delivers the necessary nutrients to cellular structures in both the dermis and epidermis. After wounding however, the endothelial cells also mediate local and systemic inflammatory responses.13 Sensory nerves, which traverse the dermis into the epidermis, also play a significant role after injury, as they mediate pain and itching, modulate inflammation, and appear to influence the remodeling phase of wound healing.14,15 The dermis, like other structures derived from mesoderm, heals not by regeneration but by fibrosis and scarring.
Pathophysiological changes of thermal injury
Applied heat at the cellular level causes denaturation of proteins and loss of plasma membrane integrity. Temperature and duration of contact have a synergistic effect, such that cell necrosis occurs after 1 s of exposure at 156°F (69°C), or after 1 h at 113°F (45°C).16 Following a burn, necrosis occurs at the center of the injury, and becomes progressively less severe at the periphery. Thus, Jackson’s description in 1953 of the three zones of injury still remains our current conceptual understanding of the burn wound (Fig. 10.1).17 The zone of coagulation is at the center of the wound where no viable cells remain. Surrounding it is the zone of stasis, characterized as a mix of viable and non-viable cells, capillary vasoconstriction, and ischemia. This tenuous area represents the zone ‘at-risk’ and may convert to necrosis with hypoperfusion, desiccation, edema, and infection. With proper wound care management however, these changes may be reversed.18 Systemic factors such as advanced age, diabetes, and other chronic illnesses also put the zone of stasis at higher risk for ‘conversion’. The outer periphery of the burn wound is the zone of hyperemia, with viable cells and vasodilation mediated by local inflammatory mediators. Tissue in this zone usually recovers completely unless complicated by infection or severe hypoperfusion.
Since medical care, for the most part, has little impact on the outcome of the zone of coagulation, efforts have focused on the prevention of necrosis in the zone of stasis. Locally, in the zone of stasis, approximately half of the cells are undergoing apoptosis versus necrosis as a result of oxidative stress, ongoing inflammation and decreased blood flow due to microthrombosis.19 It is relatively unclear if this rate of apoptosis is associated with conversion to a deeper burn; however it provides a basis for future study in elucidating techniques for healing at the zone of stasis.19 Systemically, protection of this sensitive area is achieved with adequate fluid resuscitation, avoidance of vasoconstrictors and prevention of infection.20,21 Optimal wound care consists of non-desiccating dressings, topical antimicrobials, and frequent monitoring of the wound.22–24 Interest in cooling of the wound to minimize the extent of injury can be traced to antiquity,25 but to this day, firm evidence of its efficacy is lacking. To be effective, cooling must be performed immediately after injury, and should not supersede other priorities in the evaluation of the injured patient. The optimal temperature and duration of cooling is unknown26–28; in fact, excessive or prolonged cooling may be harmful in that it promotes vasoconstriction and systemic hypothermia.29,30 Current guidelines of American Burn Association recommend limiting cooling to 30 min in the management of minor burns.31 Modalities to improve dermal perfusion and block injury from released inflammatory mediators have also garnered much interest. Experimental benefits have been reported for many pharmacologic agents such as heparin, steroidal and non-steroidal anti-inflammatory agents, thromboxane inhibitors, and epidermal growth factor.32–4019 Yet, all remain investigational since none has gained wide acceptance for clinical use.
Assessment of burn depth
Clinical observation
Burn injury may involve one or both layers of the skin, and may extend into the subcutaneous fat, muscle and even bony structures.41 Burns involving only the epidermis are erythematous and very painful but do not form blisters. Most sunburns fit this category of superficial, epidermal injury. Within 3–4 days, the dead epidermis sloughs and is replaced by regenerating keratinocytes.
Superficial dermal burns extend into the papillary dermis and characteristically form blisters. Blistering may not occur immediately following injury and burns thought to be superficial may subsequently be diagnosed as dermal burns by day.2 Once the blister is removed from a superficial partial thickness burn, the wound is pink, wet and hypersensitive to touch. Wound care is often painful as uncovering the wound allows currents of air to pass over it. These wounds blanch with pressure, and the blood flow to the dermis is increased over that of normal skin due to vasodilation. With appropriate wound care, superficial dermal burns usually heal within 2–3 weeks without risk of scarring and therefore do not require operation.
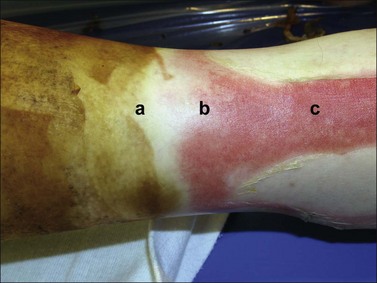
Deep dermal burns extend into the reticular dermis and generally will take 3 or more weeks to heal. They also blister, but the wound surface appears mottled pink and white immediately following the injury (Fig. 10.2). The patient complains of discomfort and pressure rather than pain. When pressure is applied to the burn, capillaries refill slowly or not at all. The wound is often less sensitive to pinprick than the surrounding normal skin. By the second day the wound may be white and is usually fairly dry. As a rule, partial-thickness burns that are predicted not to heal by 3 weeks should be excised and grafted.
The most difficult management decision involves partial-thickness burns that are intermediate in depth. In this situation, the determining factor as to whether these burns heal in 3 weeks may only be a matter of a few tenths of a millimeter. These burns are more aptly called ‘indeterminate’ burns as their healing potential becomes evident with serial assessments over several days. As evidenced by histologic studies, burn injury is a dynamic process that peaks at about 3 days.42–44 Initial evaluation by an experienced surgeon as to whether an indeterminate dermal burn will heal in 3 weeks is only about 50–70% accurate.45–47
Adjuncts to clinical evaluation
An intense search for a more precise diagnosis of burn depth has been mounted ever since it became recognized that many patients would benefit from early determination for operation. The clinical assessment of the burn wound depth is approximately 64–76% accurate in senior burn surgeons with serial exams.48 Over the last 80 years, interest in technologies for improving accurate determination of burn depth has been robust.48–50 Multiple modalities for determining burn depth have been entertained, but ultimately fallen out of favor or reinvented, which include thermography, photometry, nuclear imaging, pulse-echo ultrasound and, more invasive than the aforementioned, serial tissue biopsy.48–50 These techniques take advantage of the ability to detect: dead cells or denatured collagen (biopsy, ultrasound, vital dyes),17,51–54 the color of the wound (light reflectance),55 physical changes, such as edema (magnetic resonance imaging),56 and altered blood flow (fluorescein, laser Doppler imaging, and thermography).57–59 Unfortunately, none of these techniques has been proven superior to serial clinical assessments by an experienced burn provider. Several groups, however, have recently reported clinical benefits with the use of non-contact laser Doppler imaging in indeterminate thickness burns.44,48,60 This technique provides a color perfusion map of the burn wound to add to the clinician’s assessment. Since the scanner is held at a distance from the wound, this test is well tolerated and perhaps more reliable as no pressure is exerted on the wound. Furthermore, this test can be repeated over the first several days post-burn to document dynamic changes in wound bed perfusion. Although a promising tool, non-contact laser Doppler imaging has, so far, not been widely adopted into clinical practice.