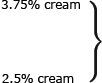Epithelial Precancerous Lesions: Introduction
A precancerous or premalignant lesion is one that has a strong likelihood of transforming into a malignancy. There is much debate about the validity of the concept of precancerous lesions, and the terminology has been confusing.1–4 Lesions discussed in this chapter are those that have a clinically demonstrated potential to become invasive carcinomas and are characterized by histologic atypia confined to the epidermis. The focus is only on the precancerous keratinocyte lesions and not on those of other epithelial cells such as the melanocyte, Merkel, and appendageal cells. Discussion of malignancies and premalignancies associated with these cells can be found in (Chapters 119, 120, and 123) respectively.
A common feature of all premalignant keratinocyte tumors (Table 113-1) is that they have the potential to become invasive squamous cell carcinoma (SCC). These precancerous lesions and SCC are considered by many to represent a continuum of disease with dysplasia at one end of the spectrum and invasive carcinoma at the other.
• Actinic keratoses (AKs) • Arsenical keratoses (ArKs) • Thermal keratoses (TKs) • Hydrocarbon keratoses (HKs) • Chronic radiation keratoses (CRKs) • Reactional keratoses (RKs) • PUVA keratoses • Viral-associated precancerous keratinocytic lesions • Bowenoid papulosis (BP) • Epidermodysplasia verruciformis (EV) • Bowen disease (BD) or squamous cell carcinoma (SCC) in situ • Precancerous lesions of the lower anogenital tract • Vulvar intraepithelial neoplasia (VIN) • Anal (AIN) and perianal (PaIN) intraepithelial neoplasia • Penile intraepithelial neoplasia (PIN) • Potentially malignant disorders of the oral cavity • Leukoplakia • Erythroplakia |
Actinic Keratoses
|
Actinic keratoses (AKs) or solar keratoses are cutaneous neoplasms consisting of proliferations of cytologically abnormal epidermal keratinocytes that develop in response to prolonged exposure to ultraviolet (UV) radiation. The concept of a precancerous keratosis was first presented by Dubreuilh in the late 1800s.5 AKs were first identified and named keratoma senilis by Freudenthal in 1926.6 In 1958, Pinkus further characterized these lesions and coined the term actinic keratosis.7 These lesions have also been called solar keratoses and senile keratoses. Actinic keratosis literally means a condition (-osis) of excessive horny (kerat-) tissue induced by a ray of light (aktis), presumably UV light. AKs have historically been considered precancerous or premalignant lesions with a potential for developing into SCCs. However, in recent years there has been an effort to redefine AKs as malignant neoplasms, because these lesions are essentially intraepithelial SCCs in evolution. Although not all AKs become SCCs, AKs are the initial lesion in a disease continuum that may progress to SCC. This concept of a progression along a spectrum is analogous to that of cervical carcinoma, for which cervical intraepithelial neoplasia (CIN) is the initial, “precancerous” lesion.8
AKs are clinically important lesions, not only because of their potential to develop into SCC, but because they are one of the strongest predictors that an individual may subsequently develop melanoma or nonmelanoma skin cancer (NMSC).9–11 With the increasing incidence and prevalence of melanomas and NMSCs (discussed in Chapters 114, 115, 123, and 124), persons with AKs are the perfect candidates for careful longitudinal observation for prevention of cutaneous malignancy and early intervention.
A joint project of the American Academy of Dermatology Association (AAD) and the Society for Investigative Dermatology (SID) reported that in 2004 the prevalence of AKs in the United States was 39.5 million, the estimated annual costs were $1.04 million, and that AKs accounted for more than 10% of all dermatology visits.12 AKs are second only to acne vulgaris as the most common reason for patients to visit a dermatologist and it is estimated that almost all persons over the age of 80 have AKs.13
Various risk factors have been identified for the expression of AKs. The two major ones are individual susceptibility and cumulative UV radiation exposure (Table 113-2). One of the most important susceptibility risk factors is age, because all the epidemiologic studies indicate that AKs increase in prevalence with increasing age, with rates ranging from less than 10% in white adults aged 20–29 years to 80% in white adults aged 60–69 years. Males appear to have more AKs than females in most epidemiologic studies, which presumably reflects greater cumulative sun exposure in males than in females.14 This gender differential is more pronounced at younger ages. Other individual susceptibility risk factors include a phenotype of fair skin that easily burns and freckles, and rarely tans; blue or light-colored eyes; and red or blond hair.14 Another individual risk factor is immunosuppression, because it is known that organ transplant recipients and patients receiving certain chemotherapy agents and possibly even biologic therapies are at increased risk of developing AKs and SCCs.15–18 In addition, persons with certain genetic syndromes, namely, albinism and xeroderma pigmentosum, and possibly Rothmund–Thomson and Bloom syndromes, are at greater risk of developing AKs.
• Individual susceptibility • Older age • Male gender • Fair skin that easily burns and freckles • Blond or red hair • Light-colored eyes • Cumulative ultraviolet radiation exposure • Immunosuppression • Prior history of actinic keratoses or other skin cancers • Genetic syndromes • Xeroderma pigmentosum • Bloom syndrome • Rothmund–Thomson syndrome |
Cumulative exposure to UV radiation, including tanning beds, is the other major risk factor for developing AKs. Evidence that sun exposure plays a role in the development of AKs is the fact that over 80% of all AKs are distributed on habitually sun-exposed areas of the body, such as the scalp, head, neck, forearms, and dorsal hands.14 Variables that affect a person’s cumulative UV radiation exposure include age, gender, occupation, recreational activities, and place of residence. As previously stated, the older the individual, the greater the prevalence of AKs and, intuitively, the greater the cumulative exposure to UV radiation. The age at which a person received the greatest amount and intensity of exposure to UV radiation appears to be significant, with such exposure in childhood apparently posing the greatest risk. In migration studies in Australia, British immigrants who moved to Australia before the age of 20 had fewer AKs than the native white Australians early in life, but they had the same rate of AKs as the native Australians in later years. British immigrants who moved to Australia after the age of 20 years never showed the same rate of AKs as the native Australians or the British immigrants who arrived at a young age.19
Although genetic and environmental factors may play a role in the development of AKs and SCC, it has long been recognized that the most important contributing factor is habitual exposure to UV radiation, namely, sunlight exposure. UV radiation is responsible for the development of AKs, and eventually SCC, in two ways: first, it causes mutations in cellular DNA that, when not repaired, lead to unrestrained growth and tumor formation; and second, it acts as an immunosuppressant preventing tumor rejection20 (see Chapters 109, 110, and 112).
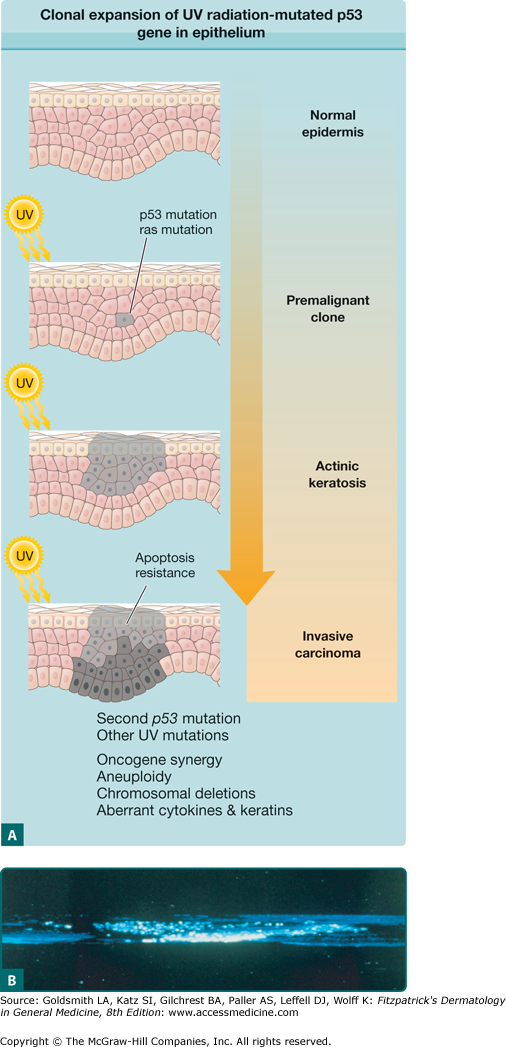
UV radiation-induced mutations in the tumor suppressor gene p53 play a pivotal role in the initiation of AKs and their development into SCC (Fig. 113-1; see Chapter 112). Multiple UV radiation-induced cellular insults to the skin result in a pathway to SCC that begins with photodamaged skin, progresses to the development of AKs, and eventually leads to some SCCs. AKs on photodamaged skin clinically represent expanded clones of genetically mutated cells that have escaped apoptosis and immune surveillance and have gone on to proliferate into clinically evident premalignant lesions.
Figure 113-1
A. Development of clonal expansion of ultraviolet (UV) radiation-mutated p53 gene in epithelium. In the multistage model of carcinogenesis, UV radiation-induced mutations provide selective growth advantage to neighboring cells, which leads to clonal expansion. (Adapted from Grossman D, Leffell DJ: The molecular basis of nonmelanoma skin cancer. Arch Dermatol 133:1263, 1997.) B. Confocal microscopy revealing expansion of UV radiation-induced clone of abnormal keratinocytes in sun-damaged skin. (From Jonason et al: Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci U S A 93:1402, 1996, with permission.)
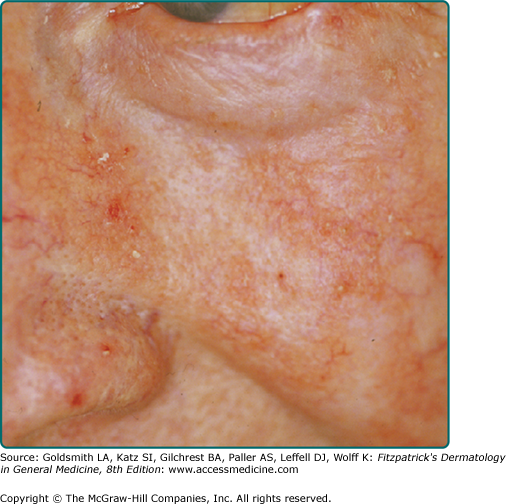
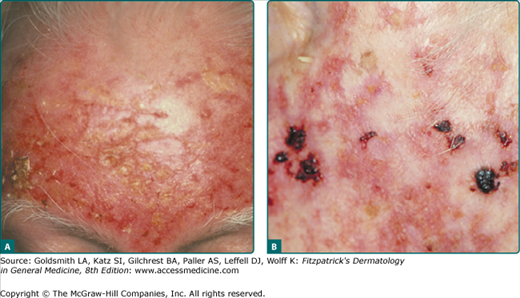
The typical patient with AKs is an older, fair-skinned, light-eyed individual who has a history of significant sun exposure, who burns and freckles rather than tans, and who has significant solar elastosis on examination (Fig. 113-2). AKs can be seen in younger individuals if these individuals have sustained sufficient sun exposure over their lives. Eighty percent of AKs are found on habitually sun-exposed sites of the body, such as the head, neck, forearms, and dorsal hands. Common signs and symptoms include pruritus, burning or stinging pain, bleeding, and crusting. The typical AK lesion, sometimes called the erythematous AK, presents most commonly as a 2–6-mm erythematous, flat, rough, gritty or scaly papule. It is usually more easily felt than seen. AKs can vary in size and sometimes reach to several centimeters in diameter. They are most often found against a background of photodamaged skin or dermatoheliosis, with solar elastosis, dyspigmentation, yellow discoloration, ephelides and lentigos, telangiectases, and sagging skin notably prominent. At times, the number and confluence of AKs are so great that the patient appears to have a rash.
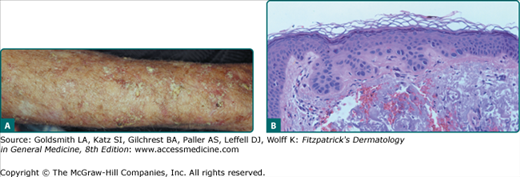
In addition to the typical erythematous AK, there are several other clinical subtypes (Table 113-3). The hypertrophic AK (HAK) presents as a thicker, scaly, rough papule or plaque that is skin-colored, gray–white, or erythematous (Fig. 113-3A). It can be found on any habitually sun-exposed site on the body but has a propensity for the dorsal hands, arms, and scalp. A typical erythematous AK can progress into an HAK. It can be difficult to distinguish an HAK from an SCC, clinically necessitating a biopsy. Biopsies must be taken to a level deep enough to ensure that the dermal extent of the keratinocytic proliferation can be evaluated in order to obtain an unequivocal histopathologic diagnosis. Induration or thickness, pain, and ulceration are the main clues to the transition of AK to SCC.
Clinical Variants of Actinic Keratoses • Erythematous actinic keratosis • Inflamed actinic keratosis • Hypertrophic actinic keratosis • Cutaneous horn • Actinic cheilitis • Pigmented actinic keratosis • Spreading pigmented actinic keratosis • Proliferative actinic keratosis • Conjunctival actinic keratosis Histopathologic Variants of Actinic Keratoses • Inflamed actinic keratosis • Hypertrophic actinic keratosis • Cutaneous horn • Atrophic actinic keratosis • Bowenoid actinic keratosis • Pigmented actinic keratosis • Proliferative actinic keratosis • Lichenoid actinic keratosis • Acantholytic actinic keratosis • Clear cell actinic keratosis |
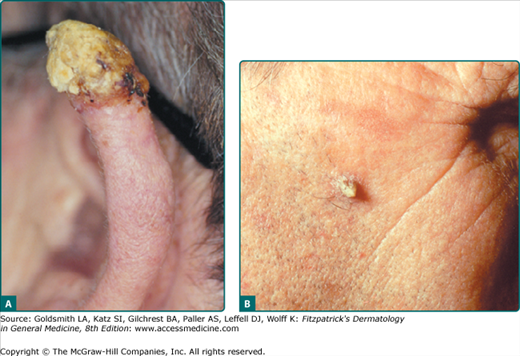
Cutaneous horn, also known as cornu cutaneum, refers to a reaction pattern and not a particular lesion (Fig. 113-4). In reference to AKs, a cutaneous horn is a type of HAK that presents with a conical hypertrophic protuberance emanating from a skin-colored to erythematous papular base. Classical definitions of a cutaneous horn maintain that the height is at least one-half of the largest diameter. Thirty-eight percent to 40% of all cutaneous horns represent AKs.21 The pathology underlying a cutaneous horn can be a number of different lesions, such as AK, SCC, seborrheic keratosis, filiform verruca vulgaris, trichilemmoma, or keratoacanthoma. The most common underlying lesion in elderly, fair-skinned persons is an HAK.
Actinic cheilitis represents confluent AKs on the lips, most often the lower lip (Fig. 113-5). Persons with this condition have red, scaly, chapped lips, and at times erosions or fissures may be present. The vermillion border of the lip is often indistinct, and focal hyperkeratosis and leukoplakia may also be seen. Individuals with this condition often complain of persistent dryness and cracking of the lips, and the diagnosis of actinic cheilitis should always be suspected in photodamaged patients with such complaints. Persistent ulcerations or indurated areas on the lip require biopsy to ensure that SCC is not present.
![]() Unusual variants of actinic keratoses (AK) include pigmented AK, spreading pigmented AK, proliferative AK, and conjunctival AK. The pigmented AK presents as a flat, tan to brown, scaly papule or plaque. Clinically, and many times dermatoscopically, it can be difficult to distinguish from a solar lentigo or lentigo maligna.22 Spreading pigmented AK is a particular lesion that is often found on the face or scalp. It is typically a large lesion, often reaching more than 1 cm in diameter, and presents as a scaly, verrucous, or smooth plaque with variable colors. Proliferative AK is seen as an expanding, red, oval, scaly plaque that reaches up to 4 cm in diameter. It has poorly defined borders. Conjunctival AK is classified as a type of pinguecula or pterygium. It appears as a wedge-shaped, opaque thickening near the limbus, and it may extend onto the cornea from the scleral conjunctiva.
Unusual variants of actinic keratoses (AK) include pigmented AK, spreading pigmented AK, proliferative AK, and conjunctival AK. The pigmented AK presents as a flat, tan to brown, scaly papule or plaque. Clinically, and many times dermatoscopically, it can be difficult to distinguish from a solar lentigo or lentigo maligna.22 Spreading pigmented AK is a particular lesion that is often found on the face or scalp. It is typically a large lesion, often reaching more than 1 cm in diameter, and presents as a scaly, verrucous, or smooth plaque with variable colors. Proliferative AK is seen as an expanding, red, oval, scaly plaque that reaches up to 4 cm in diameter. It has poorly defined borders. Conjunctival AK is classified as a type of pinguecula or pterygium. It appears as a wedge-shaped, opaque thickening near the limbus, and it may extend onto the cornea from the scleral conjunctiva.
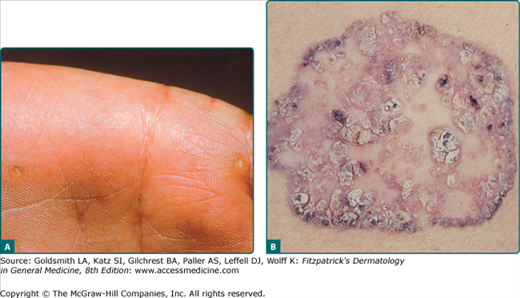
![]() The typical erythematous AK has characteristic architectural and cytologic histopathologic features. All of the abnormalities are confined to the epidermis, although dermal solar elastosis is usually present and an inflammatory dermal infiltrate may also be seen. The classic histopathologic findings include foci of atypical, pleomorphic keratinocytes along the basal cell layer and protruding as buds into the papillary dermis. The basal cell layer appears more basophilic because of crowding of the atypical keratinocytes. Overlying these foci of abnormal cells, one often sees irregular acanthosis, hyperkeratosis, and parakeratosis. There is notable sparing of the adnexal epithelium, with orthokeratosis overlying these structures, which gives rise to the characteristic pattern of alternating orthokeratosis and parakeratosis (see Fig. 113-3B). Cytologically, there is an increased nuclear–cytoplasmic ratio in the atypical keratinocytes in addition to nuclear pleomorphism and mitoses.
The typical erythematous AK has characteristic architectural and cytologic histopathologic features. All of the abnormalities are confined to the epidermis, although dermal solar elastosis is usually present and an inflammatory dermal infiltrate may also be seen. The classic histopathologic findings include foci of atypical, pleomorphic keratinocytes along the basal cell layer and protruding as buds into the papillary dermis. The basal cell layer appears more basophilic because of crowding of the atypical keratinocytes. Overlying these foci of abnormal cells, one often sees irregular acanthosis, hyperkeratosis, and parakeratosis. There is notable sparing of the adnexal epithelium, with orthokeratosis overlying these structures, which gives rise to the characteristic pattern of alternating orthokeratosis and parakeratosis (see Fig. 113-3B). Cytologically, there is an increased nuclear–cytoplasmic ratio in the atypical keratinocytes in addition to nuclear pleomorphism and mitoses.
![]() Just as there are clinical variants of AKs, there are histopathologic variants. These histopathologic variants all share the classic features of the AK as described earlier, but with some additional findings. In HAK, the hypertrophic areas consist of marked hyperkeratosis and parakeratosis. A cutaneous horn that represents AK demonstrates changes similar to but more exaggerated than those in HAKs with massive tiers of hyperkeratosis and parakeratosis. The base of such a cutaneous horn shows the typical histopathologic changes of an AK. An atrophic AK has very mild hyperkeratosis and a thinned epidermis that is devoid of rete ridges. Bowenoid AK is a histopathologic variant that demonstrates near full-thickness epidermal dysplasia, but unlike true Bowen disease (BD) or SCC in situ, there is no or very little involvement of the adnexal epithelium. In proliferative AK there are exaggerated bud-like downgrowth of atypical keratinocytes into the papillary dermis. Although difficult to distinguish from SCC at times, these proliferative bud-like downgrowth are contiguous with the epidermis in the proliferative AK, unlike in true SCC.
Just as there are clinical variants of AKs, there are histopathologic variants. These histopathologic variants all share the classic features of the AK as described earlier, but with some additional findings. In HAK, the hypertrophic areas consist of marked hyperkeratosis and parakeratosis. A cutaneous horn that represents AK demonstrates changes similar to but more exaggerated than those in HAKs with massive tiers of hyperkeratosis and parakeratosis. The base of such a cutaneous horn shows the typical histopathologic changes of an AK. An atrophic AK has very mild hyperkeratosis and a thinned epidermis that is devoid of rete ridges. Bowenoid AK is a histopathologic variant that demonstrates near full-thickness epidermal dysplasia, but unlike true Bowen disease (BD) or SCC in situ, there is no or very little involvement of the adnexal epithelium. In proliferative AK there are exaggerated bud-like downgrowth of atypical keratinocytes into the papillary dermis. Although difficult to distinguish from SCC at times, these proliferative bud-like downgrowth are contiguous with the epidermis in the proliferative AK, unlike in true SCC.
![]() In the pigmented AK one sees excessive amounts of melanin, especially in the basal cell layer. In the spreading pigmented AK variant, in addition to the typical AK histopathologic findings, one may see increased melanin in melanocytes and in the atypical keratinocytes. Lichenoid AKs are characterized by a dense, band-like lymphohistiocytic infiltrate at the dermal–epidermal junction with basal cell liquefactive degeneration and occasional Civatte bodies. In the acantholytic AK variant there are clefts of lacunae directly above the atypical keratinocytes with discohesive individual atypical keratinocytes within the clefts, reminiscent of the changes seen in Darier’s disease. The acantholysis in an acantholytic AK is a result of the anaplastic changes occurring in the lower epidermis and loss of normal keratinocytic attachments. The clear cell variant of AK arises from an excess of cytoplasmic glycogen, which results histopathologically in marked vacuolation of the epidermis.
In the pigmented AK one sees excessive amounts of melanin, especially in the basal cell layer. In the spreading pigmented AK variant, in addition to the typical AK histopathologic findings, one may see increased melanin in melanocytes and in the atypical keratinocytes. Lichenoid AKs are characterized by a dense, band-like lymphohistiocytic infiltrate at the dermal–epidermal junction with basal cell liquefactive degeneration and occasional Civatte bodies. In the acantholytic AK variant there are clefts of lacunae directly above the atypical keratinocytes with discohesive individual atypical keratinocytes within the clefts, reminiscent of the changes seen in Darier’s disease. The acantholysis in an acantholytic AK is a result of the anaplastic changes occurring in the lower epidermis and loss of normal keratinocytic attachments. The clear cell variant of AK arises from an excess of cytoplasmic glycogen, which results histopathologically in marked vacuolation of the epidermis.
![]() Two different histopathologic reaction patterns may be seen in AKs: namely, epidermolytic hyperkeratosis (EHK) and the pattern known as intraepidermal epithelioma of Borst-Jadassohn. In an otherwise histopathologically classic AK, one may find incidental changes consistent with EHK. The findings of EHK in these AKs most commonly are focal and isolated and have no clinical importance. Some authors consider intraepidermal epithelioma of Borst-Jadassohn to be a specific lesion,23 but other authors24 believe that this entity actually represents a type of reaction pattern found in a variety of lesions, such as AK, pagetoid BD, clonal seborrheic keratosis, intraepidermal malignant eccrine poroma, mammary and extramammary Paget’s disease, intraepidermal junctional nevus, and melanoma in situ. The pattern so named is that of an intraepidermal neoplasm characterized by nests and individual cells proliferating or scattered within normal epidermis. In so-called pagetoid AK, there are adjacent typical changes of AK, although differentiating this entity from pagetoid BD is often quite difficult.
Two different histopathologic reaction patterns may be seen in AKs: namely, epidermolytic hyperkeratosis (EHK) and the pattern known as intraepidermal epithelioma of Borst-Jadassohn. In an otherwise histopathologically classic AK, one may find incidental changes consistent with EHK. The findings of EHK in these AKs most commonly are focal and isolated and have no clinical importance. Some authors consider intraepidermal epithelioma of Borst-Jadassohn to be a specific lesion,23 but other authors24 believe that this entity actually represents a type of reaction pattern found in a variety of lesions, such as AK, pagetoid BD, clonal seborrheic keratosis, intraepidermal malignant eccrine poroma, mammary and extramammary Paget’s disease, intraepidermal junctional nevus, and melanoma in situ. The pattern so named is that of an intraepidermal neoplasm characterized by nests and individual cells proliferating or scattered within normal epidermis. In so-called pagetoid AK, there are adjacent typical changes of AK, although differentiating this entity from pagetoid BD is often quite difficult.
![]() An important point to remember in making the diagnosis of AK histopathologically is that if the physician has a strong clinical suspicion of SCC or basal cell carcinoma (BCC) and the initial cuts reveal AK, it is prudent to section more deeply into the block of tissue to ensure that an SCC or BCC is not missed. In one study additional diagnostic findings were present on step sections in 23 of 69 specimens (33%) initially diagnosed histopathologically as AK. Of these additional findings, 13% were indicative of BD, 4% of BCC, and 3% of invasive SCC. Three variables that correlated with the discovery of malignancy on step sections were ulceration on the first level, a clinical diagnosis of skin cancer, and a history of skin cancer confirmed previously by biopsy examination.25
An important point to remember in making the diagnosis of AK histopathologically is that if the physician has a strong clinical suspicion of SCC or basal cell carcinoma (BCC) and the initial cuts reveal AK, it is prudent to section more deeply into the block of tissue to ensure that an SCC or BCC is not missed. In one study additional diagnostic findings were present on step sections in 23 of 69 specimens (33%) initially diagnosed histopathologically as AK. Of these additional findings, 13% were indicative of BD, 4% of BCC, and 3% of invasive SCC. Three variables that correlated with the discovery of malignancy on step sections were ulceration on the first level, a clinical diagnosis of skin cancer, and a history of skin cancer confirmed previously by biopsy examination.25
![]() Because of the intimate association between AK and SCC, a number of dermatopathologists have proposed a more descriptive diagnosis that would more accurately reflect the nature of this disease. Cockerell has argued that because this entity is closely akin to cervical intraepithelial neoplasia (CIN), terms such as solar keratotic intraepidermal SCC or keratinocytic intraepidermal neoplasia would better define AK.1,8 Ackerman26 has argued that solar keratosis is in fact SCC and should not be distinguished histopathologically. At this time, actinic keratosis and solar keratosis are the most recognized terms for this diagnosis, and most authors consider these lesions precursors of SCC rather than the malignant entity itself.
Because of the intimate association between AK and SCC, a number of dermatopathologists have proposed a more descriptive diagnosis that would more accurately reflect the nature of this disease. Cockerell has argued that because this entity is closely akin to cervical intraepithelial neoplasia (CIN), terms such as solar keratotic intraepidermal SCC or keratinocytic intraepidermal neoplasia would better define AK.1,8 Ackerman26 has argued that solar keratosis is in fact SCC and should not be distinguished histopathologically. At this time, actinic keratosis and solar keratosis are the most recognized terms for this diagnosis, and most authors consider these lesions precursors of SCC rather than the malignant entity itself.
![]() The diagnosis of AK is accurately made by clinical examination in most instances when the examiner is familiar with AK and skilled in making such diagnoses. However, some investigators have commented on the difficulty of accurate and consistent counting of AK lesions by experts involved in AK research studies.27 In practice, very few AKs are histopathologically confirmed, and thus few data exist on the accuracy of clinical diagnosis. The accuracy of clinical diagnosis is higher in a clinical referral setting than in the general community setting. One study involving a referral setting revealed a diagnostic accuracy of 94%, based upon the correct histopathologic diagnosis of 34 of 36 clinically suspect, typical AKs.28 Another study in a community-based setting reported a diagnostic accuracy of approximately 80% when random clinically diagnosed AKs were subject to histopathologic confirmation.29 In the more recent multicenter Veterans Affairs Topical Tretinoin Chemoprevention Trial (VATTC), researchers found that the greatest disagreements in the histopathologic diagnosis of BCC and SCC (keratinocytic carcinomas) between two referral dermatopathologists were in making the diagnoses of AK and invasive SCC.30
The diagnosis of AK is accurately made by clinical examination in most instances when the examiner is familiar with AK and skilled in making such diagnoses. However, some investigators have commented on the difficulty of accurate and consistent counting of AK lesions by experts involved in AK research studies.27 In practice, very few AKs are histopathologically confirmed, and thus few data exist on the accuracy of clinical diagnosis. The accuracy of clinical diagnosis is higher in a clinical referral setting than in the general community setting. One study involving a referral setting revealed a diagnostic accuracy of 94%, based upon the correct histopathologic diagnosis of 34 of 36 clinically suspect, typical AKs.28 Another study in a community-based setting reported a diagnostic accuracy of approximately 80% when random clinically diagnosed AKs were subject to histopathologic confirmation.29 In the more recent multicenter Veterans Affairs Topical Tretinoin Chemoprevention Trial (VATTC), researchers found that the greatest disagreements in the histopathologic diagnosis of BCC and SCC (keratinocytic carcinomas) between two referral dermatopathologists were in making the diagnoses of AK and invasive SCC.30
![]() The clinical features of typical erythematous AKs are not unique to these lesions. The differential diagnosis includes other common lesions as noted in eBox 113-0.1. Of the clinical AK variants, pigmented AK is difficult to distinguish from solar lentigo and sometimes lentigo maligna; spreading pigmented AK, from lentigo maligna or a large seborrheic keratosis; HAK, from SCC; and cutaneous horn, from the many entities it may represent.
The clinical features of typical erythematous AKs are not unique to these lesions. The differential diagnosis includes other common lesions as noted in eBox 113-0.1. Of the clinical AK variants, pigmented AK is difficult to distinguish from solar lentigo and sometimes lentigo maligna; spreading pigmented AK, from lentigo maligna or a large seborrheic keratosis; HAK, from SCC; and cutaneous horn, from the many entities it may represent.
Most Likely
|
Always Rule Out
|
![]() Clinically, the greatest challenge is to distinguish an AK from an SCC, because the latter portends a more serious prognosis and requires different treatment. There are no reliable clinical criteria for distinguishing between these two entities, but findings of induration, pain, larger size, marked hyperkeratosis, ulceration, bleeding, rapid growth, and recurrence or persistence after treatment make the diagnosis of SCC more likely.
Clinically, the greatest challenge is to distinguish an AK from an SCC, because the latter portends a more serious prognosis and requires different treatment. There are no reliable clinical criteria for distinguishing between these two entities, but findings of induration, pain, larger size, marked hyperkeratosis, ulceration, bleeding, rapid growth, and recurrence or persistence after treatment make the diagnosis of SCC more likely.
![]() Histopathologically, AK can be confused with benign lichenoid keratosis (BLK) or lichen planus-like keratosis (LPLK), because both can show vacuolar alteration of the basal cell layer and lichenoid infiltrates. However, closer inspection reveals that in BLK/LPLK there is no cellular atypia of the keratinocytes as is seen in AK, and often the remnants of solar lentigo are seen at the periphery of the lichenoid infiltrate. An atrophic AK can be mistaken for cutaneous lupus erythematosus, because both can demonstrate epidermal thinning and basal cell liquefaction. In cutaneous lupus, however, other classic features of the disease should also be present, such as follicular plugging and a patchy periappendageal infiltrate. Finally, pigmented AK may be confused histopathologically with lentigo maligna, but the latter usually has more flattening of the epidermis, an increased number of single and nested melanocytes, atypical melanocytes, and normal keratinocytes.
Histopathologically, AK can be confused with benign lichenoid keratosis (BLK) or lichen planus-like keratosis (LPLK), because both can show vacuolar alteration of the basal cell layer and lichenoid infiltrates. However, closer inspection reveals that in BLK/LPLK there is no cellular atypia of the keratinocytes as is seen in AK, and often the remnants of solar lentigo are seen at the periphery of the lichenoid infiltrate. An atrophic AK can be mistaken for cutaneous lupus erythematosus, because both can demonstrate epidermal thinning and basal cell liquefaction. In cutaneous lupus, however, other classic features of the disease should also be present, such as follicular plugging and a patchy periappendageal infiltrate. Finally, pigmented AK may be confused histopathologically with lentigo maligna, but the latter usually has more flattening of the epidermis, an increased number of single and nested melanocytes, atypical melanocytes, and normal keratinocytes.
The prognosis of AK includes persistence, regression, or malignant transformation into invasive SCC. The relative risk of progression to SCC depends on factors related to the AK itself, such as the length of time an AK has been present and the number of baseline AKs that are on the skin. In addition, the risk for SCC increases with increased UV radiation exposure and with certain individual patient characteristics, such as suppressed immune status. Berhane et al observed that before an AK progresses to SCC it may become clinically tender and inflamed. Histopathologic examination of such clinically inflamed AKs revealed the actual presence of overt SCC in 50%.31 Thus, pain and inflammation in an AK may be a marker of progression to SCC.
Several studies have attempted to determine the risk for the progression of AK to SCC, but most of them are inadequate in one respect or another. The risk for progression of AK to SCC reported in the literature varies from less than 1%32 to 20%.33 In a review of five clinical research studies carried out between 1988 and 1998, Glogau found that the published risk of progression of AK to SCC for individual lesions ranged from 0.025% to 16% per year.34 Extrapolation from these clinical studies suggests a rate of risk of progression of AK to SCC of approximately 8%, computed as an average of the cited statistical rates.34 A more recent study by Criscione et al found that the risk of progression of AK to primary SCC (invasive or in situ) was 0.60% at 1 year and 2.57% at 4 years.35 The risk of malignant transformation can also be assessed by evaluating the percentage of SCCs that arise from preexisting AKs. Several older studies have approached this issue by histopathologically examining invasive SCC specimens and determining the percentage of cases in which an associated or contiguous AK could be identified. An associated AK was identified at the periphery or within the SCC in 60%,32 82.4%,36 97%,37 97.2%,38 and 100%39 of reviewed cases in various studies. There has been one prospective study in which all SCCs treated by the authors in one calendar year were evaluated histopathologically to see if an AK was present in the specimen. They found a contiguous AK within a histopathologically confirmed SCC in 72% of cases.40 The most recent 2009 VATTC trial by Criscione et al showed that the majority of primary SCCs (65%) and, interestingly, 36% of all primary BCCs arose within a previously clinically diagnosed AK.35 Their findings that a sizeable number of initially clinically identified AKs were later determined to be BCCs at follow-up translated into a risk of progression of 0.48% at 1 year and 1.56% at 4 years. The authors commented that it was not clear if the initially identified AKs developed into BCCs, a phenomenon that has never before been reported, or if the BCCs were previously misidentified as AKs. We have to assume the latter, as BCCs originate from the follicular germ cells and are distinct from AK/SCC.41
Their conclusion, however, was that there was an almost linear increased risk of clinically diagnosed AKs progressing to either SCC or BCC at 4 years, demonstrating that AKs have a significant role in the overall burden of keratinocyte carcinomas and are an important marker for the development of NMSC.35
Spontaneous regression of AKs has been reported, but it is impossible to predict for any particular AK lesion.35,42,43 One study reported that up to 25% of AKs remitted over a 1-year period, especially if sun exposure was limited during that time.44 Another study found that more AKs regressed in individuals who routinely wore sunscreen.29 The 2009 VATTC study reported that 55% and 70% of AKs followed clinically were not present at 1-year and 5-year follow-up points, respectively.35 However, the authors noted that most importantly the vast majority (87%) of AK lesions identified at the 1- and 5-year visits were not clinically identified during at least one of the 6-month intervals, suggesting that AKs come and go.35
The presence of AKs indicates long-term sun damage and identifies a group of people who are at high risk for developing SCC, BCC, and, to a lesser extent, melanoma.9–11
The estimated rates of metastases arising from actinically derived SCC have ranged from 1% to 2% to over 20% depending primarily on depth of invasion, location (with the lip, ear, and scalp representing the sites of highest risk), differentiation, and the presence of perineural invasion.45
The inability to predict which AKs will persist, regress, or become SCCs makes treatment of these lesions equally confusing. Although some clinicians have argued that because of the low malignant transformation risk, treatment of AKs is unnecessary, most dermatologists advocate the treatment of AKs to avoid any chance of progression to invasive SCC.46,47 The 2009 VATTC trial researchers concluded from their findings that despite the possibility of AK regression, new AKs and keratinocyte carcinomas can develop and that active treatment of AK lesions is warranted, especially given the overall contribution of AKs to SCC and BCC burden.35 Treatment of this disease is also warranted to minimize symptoms, such as pain and pruritus, in affected patients.
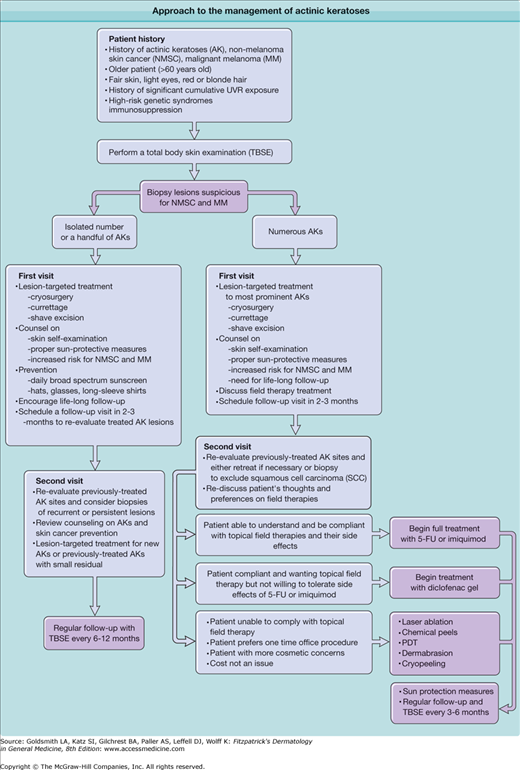
In selection of the proper treatment, there are no absolute guidelines or algorithms because published studies vary considerably in their design, measured outcomes, and follow-up time (Fig. 113-6). The clinical diagnosis of AK even by expert dermatologists can be inconsistent.27
Treatment modalities for AKs can be broadly divided into lesion-targeted therapies and field therapies (Box 113-1).
LESION-TARGETED THERAPIES
|
FIELD THERAPIES
|
The majority of the lesion-targeted therapies for the treatment of AKs are destructive, i.e., they work by physically removing the AK. Such destructive treatments are currently the most common methods used to treat AKs.48
Liquid nitrogen cryosurgery is the most common destructive procedure and is typically administered with a spray device or a cotton-tipped applicator (see Chapter 246). The first prospective study looking at efficacy rates of cryosurgery was performed in 2004.49 The overall complete clearance rate when the patients were checked 3 months after treatment was 67.2%. A subgroup analysis of these data based on actual freeze times indicated that a complete response occurred in 39% of cases with freeze times of 5 seconds or less, in 69% with freeze times between 6 seconds and 20 seconds, and in 83% with freeze times longer than 20 seconds. Hypopigmentation was seen in 29% of sites from which AK was eradicated most probably those with longer freeze times as the melanocyte is particularly susceptible to cold injury. The investigators concluded that the ideal freeze time was somewhere between 10 seconds and 15 seconds.
The benefits of cryosurgery are its ease of administration in trained hands, the lack of need for anesthetic, and the lack of reliance on patient compliance other than in posttreatment care of treated lesions. Potential disadvantages of cryosurgery include pain and discomfort, the presence of unsightly blisters and crusted wounds for a week or longer, hypopigmentation, scarring, and possible alopecia in treated areas.50 In rare cases, serious injury to underlying tendons and nerves may occur with deep or prolonged liquid nitrogen application to the hands. Most important, cryosurgery is best used to treat a limited number of clinically perceptible or symptomatic lesions.
Curettage, with or without electrosurgery (see Chapter 246), is another destructive lesion-targeted treatment for AKs. This procedure and cryosurgery together constitute approximately 80% of all treatments for AKs in the United States.48 A curette is used to mechanically scrape away the atypical keratinocytes comprising the AK. Electrosurgery may or may not be used to further destroy atypical cells and to provide hemostasis. If electrosurgery is employed, minimal use is advised to enhance the final cosmetic result. A local anesthetic is needed for this procedure, and hemostatic agents such as aluminum chloride can be used to stop the bleeding if electrosurgery is not utilized. There are no controlled studies addressing cure rates with the use of this technique for the treatment of AKs, but experience says it is quite effective, at the expense of potential scarring. Patients can expect some discomfort with injection of the local anesthetic, and the treated area will take a few weeks to heal completely. This technique is most appropriate for patients with relatively few AKs. It is also beneficial for treatment of lesions after biopsy and for the treatment of HAKs. Potential side effects include infection, scarring, and dyspigmentation. If a biopsy is to be performed, procurement of a shaved specimen before curettage provides a much more acceptable histopathologic portrait and avoids overinterpretation or underinterpretation. Obtaining biopsy samples through curettage produces crushed and fragmented specimens that are difficult to interpret, which can lead to erroneous diagnoses.
The third lesion-targeted destructive therapy for AKs is shave excision. This technique involves injection of a local anesthetic followed by tangential excision of the lesion with a surgical blade (see Chapter 243). Hemostatic agents must also be used to stop the bleeding. No data exist on the cure rate of this technique, but as with curettage, anecdotal experience says it is effective. Healing time may be 1–2 weeks, and potential complications include infection, scarring, and dyspigmentation. Use of this technique is most often indicated when a clinically apparent AK is suspicious for SCC or BCC and histopathologic examination is needed. Shave excision offers the patient an attempt at curative therapy simultaneous with a diagnostic procedure. Signs and symptoms that should arouse suspicion of SCC include marked erythema, pain, ulceration, bleeding, induration, or failure to respond to prior destructive therapies. Care must be taken to shave deeply enough to avoid transecting the AK at the deep margin, because this precludes unequivocal interpretation due to the proclivity for invasive SCC to develop at the deepest extensions of the atypical keratinocytes in AKs.
Field therapies treat larger areas of photodamaged skin that contain both clinical and subclinical AK lesions. Field therapies can be further categorized into topical/medical and procedural field therapies. Such treatments are best used in patients with moderate to severely photodamaged skin and numerous AKs that would be too burdensome and painful to treat with the lesion-targeted therapies. Many patients with AKs, upon close inspection, are found to be extensively affected with early disease in sun-exposed areas, which may make field therapy a more rational approach to this difficult problem.
Several topical agents are available and approved by the US Food and Drug Administration (FDA) for the generalized treatment of AKs, including 5-fluorouracil (5-FU), 5% imiquimod cream, and 3% diclofenac gel (Box 113-2).
MEDICATION | TOPICAL PREPARATION | DOSAGE |
|---|---|---|
5-Fluorouracil | 2% solution 5% solution 1% cream 5% cream 0.5% micronized cream | Twice daily for up to 4 weeks Once daily for 4 weeks |
Imiquimod | 5% cream
| Twice per week for 16 wks daily for 2 wks, then no treatment for 2 wks, then daily for 2 weeks |
Diclofenac | 3% gel | Twice daily for 90 days |
![]() 5-FU therapy has been a mainstay of topical treatment for AKs since the 1960s (see Chapter 220). 5-FU is a pyrimidine analog that inhibits the enzyme, thymidylate synthase, thus, interfering in DNA synthesis and ultimately resulting in apoptosis and tumor cell death. 5-FU acts selectively to cause cellular destruction in actinically damaged cells but not in normal skin. 5-FU treatment of sun-damaged skin usually causes a brisk inflammatory reaction, which is felt to contribute to its overall efficacy.
5-FU therapy has been a mainstay of topical treatment for AKs since the 1960s (see Chapter 220). 5-FU is a pyrimidine analog that inhibits the enzyme, thymidylate synthase, thus, interfering in DNA synthesis and ultimately resulting in apoptosis and tumor cell death. 5-FU acts selectively to cause cellular destruction in actinically damaged cells but not in normal skin. 5-FU treatment of sun-damaged skin usually causes a brisk inflammatory reaction, which is felt to contribute to its overall efficacy.
![]() Although many different treatment regimens have been proposed and used with topical 5-FU, the historical standard FDA-approved method consists of twice-daily application of 5-FU cream or solution to the entire affected region, typically for 2–4 weeks. Spot treatment of specific AK lesions is not recommended but is a common practice. There is also some evidence that concurrent treatment or pretreatment with topical tretinoin is more effective than 5-FU alone for AKs on the extremities.51 Therapy should be continued until the treated area demonstrates erythema, erosion, crusting, and necrosis, at which time treatment should be discontinued. Different strengths (1%, 2%, 5%) and vehicles (cream or solution) of topical 5-FU are available. More recently, a 0.5% micronized 5-FU cream has become available and FDA approved for treating AKs. It can be applied once daily for 4 weeks. The choice of which topical 5-FU formulation to use depends on physician preference, individual patient characteristics, and the number and location of AKs being treated.
Although many different treatment regimens have been proposed and used with topical 5-FU, the historical standard FDA-approved method consists of twice-daily application of 5-FU cream or solution to the entire affected region, typically for 2–4 weeks. Spot treatment of specific AK lesions is not recommended but is a common practice. There is also some evidence that concurrent treatment or pretreatment with topical tretinoin is more effective than 5-FU alone for AKs on the extremities.51 Therapy should be continued until the treated area demonstrates erythema, erosion, crusting, and necrosis, at which time treatment should be discontinued. Different strengths (1%, 2%, 5%) and vehicles (cream or solution) of topical 5-FU are available. More recently, a 0.5% micronized 5-FU cream has become available and FDA approved for treating AKs. It can be applied once daily for 4 weeks. The choice of which topical 5-FU formulation to use depends on physician preference, individual patient characteristics, and the number and location of AKs being treated.
![]() Anecdotally, topical 5-FU has been thought to be 75% effective, that is, seven or eight out of ten treated AKs will resolve with appropriate therapy.52 The long-term remission rates after 5-FU treatment vary, but the condition frequently recrudesces in a few years and severely affected patients sometimes return to baseline.
Anecdotally, topical 5-FU has been thought to be 75% effective, that is, seven or eight out of ten treated AKs will resolve with appropriate therapy.52 The long-term remission rates after 5-FU treatment vary, but the condition frequently recrudesces in a few years and severely affected patients sometimes return to baseline.
![]() The once-daily, 0.5% micronized 5-FU cream was developed in an attempt to make the topical 5-FU therapy more tolerable. It is FDA approved for the treatment of multiple AKs on the face and anterior scalp. Premarketing studies of this medication showed that more than 75% of treated lesions clinically resolved in 60%–80% of patients treated for 4 weeks. One study found an overall 89% reduction of AKs and a complete clearance rate of 48% in patients treated for 4 weeks with a once-daily application of 0.5% micronized 5-FU cream.53 In a split-face comparison study of 5% 5-FU and 0.5% 5-FU cream, similar complete clearance rates of approximately 50% were observed for both therapies.54 The investigators noted a similar reaction rate with erythema, crusting, and scabbing, but overall the patients preferred the 0.5% micronized 5-FU cream because of its once-daily application regimen and a perception of less irritation.
The once-daily, 0.5% micronized 5-FU cream was developed in an attempt to make the topical 5-FU therapy more tolerable. It is FDA approved for the treatment of multiple AKs on the face and anterior scalp. Premarketing studies of this medication showed that more than 75% of treated lesions clinically resolved in 60%–80% of patients treated for 4 weeks. One study found an overall 89% reduction of AKs and a complete clearance rate of 48% in patients treated for 4 weeks with a once-daily application of 0.5% micronized 5-FU cream.53 In a split-face comparison study of 5% 5-FU and 0.5% 5-FU cream, similar complete clearance rates of approximately 50% were observed for both therapies.54 The investigators noted a similar reaction rate with erythema, crusting, and scabbing, but overall the patients preferred the 0.5% micronized 5-FU cream because of its once-daily application regimen and a perception of less irritation.
![]() Effective treatment relies greatly on the patient’s understanding and compliance with the prescribed 5-FU regimen. The clinician should spend time with the patient educating the patient about the proper application of the medication, the duration of therapy, and the expected outcome. Written handouts reiterating this information and photographs of the expected reaction are helpful for patients to view and take home.
Effective treatment relies greatly on the patient’s understanding and compliance with the prescribed 5-FU regimen. The clinician should spend time with the patient educating the patient about the proper application of the medication, the duration of therapy, and the expected outcome. Written handouts reiterating this information and photographs of the expected reaction are helpful for patients to view and take home.
![]() Patients should expect discomfort, pruritus, and burning at the sites of application, as well as erythema, erosion, crusting, and possibly ulceration beginning at around 1 week into treatment (eFig. 113-6.1). These and other adverse reactions to 5-FU are further discussed in Chapter 220. Other photodamaged areas of the skin that seemingly have no visible evidence of AKs may also become quite inflamed during treatment, leading not only to the treatment of unmasked AKs, but also to the histopathologically proven rejuvenation of the skin.55 Much lower cure rates are seen when patient compliance is poor and the expected end points are not reached.
Patients should expect discomfort, pruritus, and burning at the sites of application, as well as erythema, erosion, crusting, and possibly ulceration beginning at around 1 week into treatment (eFig. 113-6.1). These and other adverse reactions to 5-FU are further discussed in Chapter 220. Other photodamaged areas of the skin that seemingly have no visible evidence of AKs may also become quite inflamed during treatment, leading not only to the treatment of unmasked AKs, but also to the histopathologically proven rejuvenation of the skin.55 Much lower cure rates are seen when patient compliance is poor and the expected end points are not reached.
![]() The second agent available for topical field treatment of multiple AKs is imiquimod cream (see Chapter 220). It works by inducing both the innate and adaptive arms of the immune system to recognize and destroy AKs. It is available in 5%, 3.75% and 2.5% formulations. First approved in 1997 by the FDA for the treatment of genital and perianal warts, it was approved in 2004 for the treatment of nonhypertrophic AKs on the face and scalp in immunocompetent adults. It is also approved for treatment of superficial BCC (see Chapter 115).
The second agent available for topical field treatment of multiple AKs is imiquimod cream (see Chapter 220). It works by inducing both the innate and adaptive arms of the immune system to recognize and destroy AKs. It is available in 5%, 3.75% and 2.5% formulations. First approved in 1997 by the FDA for the treatment of genital and perianal warts, it was approved in 2004 for the treatment of nonhypertrophic AKs on the face and scalp in immunocompetent adults. It is also approved for treatment of superficial BCC (see Chapter 115).
![]() The FDA-approved regimens for the three different strengths of imiquimod are outlined in Box 113-2. Treatment with imiquimod cream is associated with adverse reactions, most notably local skin reactions such as erythema, scabbing, flaking, erosion, weeping, and vesicle formation. Such reactions are actually predictive of better ultimate clearance of the AKs, as the greatest resolution of AKs has been seen in patients with the most severe reactions.56–58 Systemic absorption is minimal, and overall excellent safety margins have been demonstrated with the topical administration of imiquimod cream.59 Systemic adverse effects, including interferon-like side effects such as flu-like symptoms, headaches, and myalgia, have been infrequently reported particularly when large surface areas are treated.
The FDA-approved regimens for the three different strengths of imiquimod are outlined in Box 113-2. Treatment with imiquimod cream is associated with adverse reactions, most notably local skin reactions such as erythema, scabbing, flaking, erosion, weeping, and vesicle formation. Such reactions are actually predictive of better ultimate clearance of the AKs, as the greatest resolution of AKs has been seen in patients with the most severe reactions.56–58 Systemic absorption is minimal, and overall excellent safety margins have been demonstrated with the topical administration of imiquimod cream.59 Systemic adverse effects, including interferon-like side effects such as flu-like symptoms, headaches, and myalgia, have been infrequently reported particularly when large surface areas are treated.
![]() The short-term efficacy of imiquimod cream for eradicating AKs has been well studied in the last several years. The first systematic review and meta-analysis of short-term randomized controlled trials evaluating its efficacy and safety has been published.60 For this meta-analysis, five trials57,58,61–63 involving a total of 1,293 patients were selected for inclusion based on their high-quality scores, minimal risk of bias, validity, adequate treatment duration, inclusion of patients with multiple AK lesions, and use of at-home application. Complete clearance occurred in 50% of patients treated with imiquimod versus 5% treated with vehicle alone. Partial clearance (more than 75% of lesions) was seen in 65% of imiquimod-treated patients versus 11% of vehicle-treated patients.60
The short-term efficacy of imiquimod cream for eradicating AKs has been well studied in the last several years. The first systematic review and meta-analysis of short-term randomized controlled trials evaluating its efficacy and safety has been published.60 For this meta-analysis, five trials57,58,61–63 involving a total of 1,293 patients were selected for inclusion based on their high-quality scores, minimal risk of bias, validity, adequate treatment duration, inclusion of patients with multiple AK lesions, and use of at-home application. Complete clearance occurred in 50% of patients treated with imiquimod versus 5% treated with vehicle alone. Partial clearance (more than 75% of lesions) was seen in 65% of imiquimod-treated patients versus 11% of vehicle-treated patients.60
![]() Evidence also supports the use of imiquimod in the treatment of AKs to promote long-term immune surveillance and lower recurrence rates. Stockfleth et al56 followed 25 imiquimod-treated patients from a previous short-term study61 for up to 2 more years. Of these 25 patients (one was lost to follow-up), 8% (n = 2), 16% (n = 4), and 20% (n = 5) developed new AKs or were lost to follow-up at 12, 18, and 24 months, respectively, after completion of the initial regimen of imiquimod application three times a week for 12 weeks. No patients had developed SCC in the imiquimod-treated areas at 2 years after treatment. Ten of the vehicle-treated patients from the original study61 were also observed for 1 year after their initial treatment. In this group, 90% (n = 9) developed new AKs in the vehicle-treated area, 10% (n = 1) had spontaneous remission of their AKs, and 10% (n = 1) developed an SCC in the vehicle-treated area.
Evidence also supports the use of imiquimod in the treatment of AKs to promote long-term immune surveillance and lower recurrence rates. Stockfleth et al56 followed 25 imiquimod-treated patients from a previous short-term study61 for up to 2 more years. Of these 25 patients (one was lost to follow-up), 8% (n = 2), 16% (n = 4), and 20% (n = 5) developed new AKs or were lost to follow-up at 12, 18, and 24 months, respectively, after completion of the initial regimen of imiquimod application three times a week for 12 weeks. No patients had developed SCC in the imiquimod-treated areas at 2 years after treatment. Ten of the vehicle-treated patients from the original study61 were also observed for 1 year after their initial treatment. In this group, 90% (n = 9) developed new AKs in the vehicle-treated area, 10% (n = 1) had spontaneous remission of their AKs, and 10% (n = 1) developed an SCC in the vehicle-treated area.
![]() In a second study of long-term efficacy, Lee et al64 looked at patients from four previous phase III randomized, vehicle-controlled studies who showed complete clearance of all of the AKs in the treated areas at 8-weeks posttreatment. After a median follow-up of 16 months they found that 25% (n = 19/77) and 43% (n = 23/54) of patients treated with imiquimod three times per week and two times per week, respectively, for a duration of 16 weeks experienced a recurrence of one or more AKs in original treated area.
In a second study of long-term efficacy, Lee et al64 looked at patients from four previous phase III randomized, vehicle-controlled studies who showed complete clearance of all of the AKs in the treated areas at 8-weeks posttreatment. After a median follow-up of 16 months they found that 25% (n = 19/77) and 43% (n = 23/54) of patients treated with imiquimod three times per week and two times per week, respectively, for a duration of 16 weeks experienced a recurrence of one or more AKs in original treated area.
![]() Imiquimod has been shown to be safe and effective in treating AKs in the organ transplant population, with no evidence of graft rejection and no trends toward diminished graft functioning.65 Rare flares of underlying autoimmune disease were observed during preapproval research studies and have been documented in the literature.66
Imiquimod has been shown to be safe and effective in treating AKs in the organ transplant population, with no evidence of graft rejection and no trends toward diminished graft functioning.65 Rare flares of underlying autoimmune disease were observed during preapproval research studies and have been documented in the literature.66
![]() The third FDA-approved topical field therapy for treating AKs is 3% diclofenac gel. Diclofenac is a nonsteroidal anti-inflammatory drug whose mechanism of action is not completely understood but is thought to involve inhibition of cyclooxygenase (COX), a rate-limiting enzyme in the synthesis of prostaglandins. Diclofenac appears to have greater affinity for the COX-2 enzyme than for the COX-1 isoform. One theory regarding its role in eradicating AKs relates to the finding that the production of prostaglandins from arachidonic acid may contribute to UV radiation-induced NMSC. Thus, inhibition of this cascade by diclofenac may also explain its efficacy in treating AKs.67
The third FDA-approved topical field therapy for treating AKs is 3% diclofenac gel. Diclofenac is a nonsteroidal anti-inflammatory drug whose mechanism of action is not completely understood but is thought to involve inhibition of cyclooxygenase (COX), a rate-limiting enzyme in the synthesis of prostaglandins. Diclofenac appears to have greater affinity for the COX-2 enzyme than for the COX-1 isoform. One theory regarding its role in eradicating AKs relates to the finding that the production of prostaglandins from arachidonic acid may contribute to UV radiation-induced NMSC. Thus, inhibition of this cascade by diclofenac may also explain its efficacy in treating AKs.67
![]() The FDA-approved regimen for diclofenac in the treatment of AKs is twice-daily application for 90 days. Compared with imiquimod and 5-FU, diclofenac does not cause as much inflammation, irritation, or downtime for the patient. Adverse effects include mild local site reactions, such as erythema, pruritus, flaking, and possible contact dermatitis. In a blinded, controlled study involving 96 patients complete clearance of AKs was observed in 50% of the diclofenac-treated group and in 20% of the vehicle-treated group 30 days after the conclusion of therapy.68 At 1-year follow-up, 91% of patients had at least 75% clearance of the targeted lesions, and of these patients, 79% showed 100% clearance.68
The FDA-approved regimen for diclofenac in the treatment of AKs is twice-daily application for 90 days. Compared with imiquimod and 5-FU, diclofenac does not cause as much inflammation, irritation, or downtime for the patient. Adverse effects include mild local site reactions, such as erythema, pruritus, flaking, and possible contact dermatitis. In a blinded, controlled study involving 96 patients complete clearance of AKs was observed in 50% of the diclofenac-treated group and in 20% of the vehicle-treated group 30 days after the conclusion of therapy.68 At 1-year follow-up, 91% of patients had at least 75% clearance of the targeted lesions, and of these patients, 79% showed 100% clearance.68
The second category of field therapies for treating diffuse AKs is the procedural field therapies. These include cryopeeling (see Chapter 246), dermabrasion, medium and deep chemical peels (see Chapter 251), laser resurfacing (see Chapter 251), and photodynamic therapy (PDT) (see Chapter 238).
Cryopeeling consists of extensive liquid nitrogen cryosurgery to the areas of discrete AKs as well as to the surrounding actinically damaged skin.
Dermabrasion is an older technique that is quite effective in the treatment and prophylaxis of AKs but is now rarely used for this purpose. It causes physical destruction of the AKs with abrasion using either drywall sanding sheets or dermabrasion diamond fraises, which are powered or handheld (see Chapter 251).
Medium-depth chemical peels using Jessner’s solution and 35% trichloroacetic acid (TCA) are moderately effective in treating diffuse AKs, especially when a series of such peels are administered over time69,70 (see Chapter 251). A combined peel, in which 35% TCA is used, after applied to obtain uniform frosting after Jessner’s solution degreasing and deepithelialization, has been shown to be equivalent in efficacy to 5-FU therapy.71
Deep chemical peeling using phenol or high concentrations of TCA is more effective in treating thick AKs or those with appendageal epithelial atypia but are rarely used because of the potential cardiac and renal toxicity of phenol, the greater risk of scarring and infection, and the dramatic hypopigmentation that may occur postoperatively.
Laser resurfacing for the treatment and prophylaxis of AKs is another procedural field therapy that has been utilized. The carbon dioxide (CO2) laser and the erbium:yttrium-aluminum-garnet (er:YAG) laser are the two laser systems that have primarily been investigated for these purposes (see Chapter 252). Both of these devices ablate the epidermis at varying depths allowing reepithelialization with adnexal keratinocytes that are less actinically damaged.
In preliminary small series, both laser resurfacing systems have been reported to be effective in short-term clearing of multiple facial and scalp AKs72–74 and in the long-term prevention of recurrences75 and possibly the development of NSMC.72 Laser resurfacing is probably best reserved for use by specially trained and experienced physicians and for patients with more cosmetic concerns or with diffuse AKs for which topical field therapies and/or chemical peeling have failed. They are also excellent modalities for patients with severe actinic cheilitis and for those patients with multiple, thicker AKs.
PDT is another procedural therapy available for the treatment of multiple and diffuse AKs. The FDA has approved two PDT systems for the treatment of non-HAK lesions on the face and scalp (see Chapter 238). The first to receive approval was the combination of 5-aminolevulinic acid (ALA) with a blue light source in 1998. More recently, the FDA approved a system that has been widely available in Europe, combining a methyl ester of aminolevulinic acid (MAL) with a red light source. The topically applied ALA or MAL accumulates preferentially in the more rapidly dividing atypical cells and is converted sequentially to protoporphyrin IX, a heme precursor and photosensitizer. When the ALA or MAL-treated skin is then exposed to a light source of the appropriate wavelength several hours later, the accumulated protoporphyrin IX generates a phototoxic reaction that destroys the treated cells.76–79
Randomized, placebo-controlled studies have demonstrated the efficacy of both ALA and MAL PDT for the treatment of AKs.79–82 A more recent, randomized, double-blind, prospective study comparing the safety and efficacy of ALA-PDT with MAL-PDT found both forms to have no significant difference in efficacy of decreasing AK lesion burden, but that ALA-PDT was associated with more painful and adverse effects and a longer duration of discomfort compared with MAL-PDT.82 Patients can expect some discomfort with PDT, including erythema, edema, and burning or stinging during exposure to the light source. In a subgroup of patients with severe sun damage, PDT can create intolerable pain that sometimes results in premature discontinuation of treatment. Allergic reactions to ALA and MAL have also been reported. Severe phototoxic reactions can occur if patients do not follow the protocol and practice strict sun avoidance for the recommended period after ALA and MAL application. Cosmetic outcomes have been good to excellent in those who complete adequate treatment.
There is a paucity of comparative effectiveness research amongst these various field therapies for AKs. In general, topical 5-FU, 5% imiquimod cream, diclofenac gel, and PDT are all effective in decreasing the AK lesion burden. From the mostly small comparative studies that have been published, a few additional points can be made. Topical 5-FU and topical 5% imiquimod cream seem to create more inflammation, irritation, and patient discomfort compared with topical diclofenac gel. There is some data to suggest that topical 5% imiquimod cream may have lower, long-term recurrence rates of AKs, in part by creating longer term immune memory and antitumor response.56,83 A pharmacoeconomic study of AK field treatment modalities (5% imiquimod vs. diclofenac gel vs. 5-FU vs. ALA-PDT) in combination with liquid nitrogen cryosurgery found that ALA-PDT was the least costly and that imiquimod was the most expensive.84
In summary, evidence supports the treatment of AKs to prevent the progression to malignancy. There are a number of effective lesion targeted and field treatments available to choose from to decrease the burden of AKs. Consideration of the individual’s needs, the physician’s skills, the mechanisms of action of the various treatments and their side effect profiles, plus the cost of treatment should all be considered when choosing a treatment strategy. To help physicians in choosing a treatment strategy for their patients, the American Academy of Dermatology and the European Dermatology Forum has proposed guidelines for the management of AKs, based upon available evidence at the time of their writings.85,86 In addition, the authors of this chapter have proposed a treatment algorithm (Fig. 113-6).
![]() The future of treatment for AKs is an area of great interest and research. Investigators are looking at reformulating existing topical agents to improve upon efficacy and to decrease adverse side effects. Older methods are being challenged in clinical studies with newer regimens that involve sequential or concurrent therapy with existing AK treatments. Another interesting area of research is looking at the various combinations of lesion-directed therapies with field treatments, and lastly, new emerging agents are undergoing rigorous clinical research to establish their efficacy and safety.87–89
The future of treatment for AKs is an area of great interest and research. Investigators are looking at reformulating existing topical agents to improve upon efficacy and to decrease adverse side effects. Older methods are being challenged in clinical studies with newer regimens that involve sequential or concurrent therapy with existing AK treatments. Another interesting area of research is looking at the various combinations of lesion-directed therapies with field treatments, and lastly, new emerging agents are undergoing rigorous clinical research to establish their efficacy and safety.87–89
Given the potential of AKs to progress to malignant lesions, it is important to focus on the prevention of these precancerous lesions. Educational and preventive efforts should be directed toward children, targeted high-risk populations, and all patients. Avoidance of UV radiation is the single most effective means of decreasing the risk of AKs. A strategy designed to limit the amount and intensity of sun exposure, starting in childhood and continuing throughout the person’s life, will likely decrease the number of AKs an individual will develop. Because complete avoidance of the sun is impractical, the next best preventive measures are to avoid exposure to intense midday sun; consistently apply and reapply broad-spectrum sunscreens; wear UV-protective clothing, hats, and sunglasses; install UV-protective windows where indicated; and make sure to take an oral vitamin D supplement if necessary to avoid vitamin D insufficiency or deficiency.90
Numerous randomized studies have shown that the use of sunscreen can decrease the incidence and prevalence of AKs, reduce the number of AK lesions, and increase their rate of regression.29,91–94 There is also evidence that sunscreen use can prevent certain types of skin cancer, mostly SCC.93,94 There is limited evidence that adhering to a diet low in fat may decrease the incidence of AKs95,96 and NMSC.97,98 In older studies, topical retinoids were shown to reduce the number of AKs after long-term consistent use,99,100 and in another study, long-term treatment with topical retinoic acid was shown to reduce keratinocyte and melanocyte atypia in photoaged skin.101 However, the more recent multicenter VATTC trial found that up to twice-daily application of 0.1% tretinoin cream over 1.5–5.5 years in mostly elderly (n = 1,131, mean age = 70 years) males (95%) at very high risk for NMSC was ineffective for preventing BCC and SCC.102 This study was stopped 6 months prematurely because of excess mortality in the treatment group. However, the authors could not establish a cause and effect relationship.103 They also concluded that retinoids may have very different clinical effects when administered topically as opposed to orally.102
Unlike the controversy with topical retinoids, there is strong evidence for the use of systemic retinoids in preventing NMSC and AKs, especially in high-risk populations, such as organ transplant recipients, patients with xeroderma pigmentosum, and other chronically immunosuppressed patients.104–107 Unfortunately, the systemic retinoids are only effective while the patient is taking them and their use is also limited by the frequent occurrence of systemic toxicities, including hypercholesterolemia, hypertriglyceridemia, mucocutaneous xerosis, musculoskeletal abnormalities, and alteration in liver function. Thus, when considering the use of systemic retinoids in such high-risk patients, one must weigh these risks and benefits.
Topical imiquimod has also been safely used in the organ transplant population to prevent the development of cutaneous SCC.65,108
Arsenical Keratoses
|
Arsenical keratoses (ArKs) are precancerous lesions found in association with chronic arsenicism. These lesions have the potential to develop into invasive SCC. Arsenic is a ubiquitous element that has no color, taste, or odor. It has the potential to cause characteristic acute and chronic syndromes in persons exposed to it, and such exposures are typically obscure because medicinal, occupational, and environmental sources still exist. Detection of acute and chronic arsenicism is important, because the acute form can be fatal and the chronic form is associated with a variety of cutaneous and internal malignancies. ArKs are associated with chronic arsenicism.
![]() Knowledge of the medicinal benefits and poisoning potential of arsenic dates back to ancient times. Arsenic was introduced into the United States Pharmacopoeia in 1850, and before its use was discontinued around 1965, it was employed medicinally in the United States and Europe in the form of Fowler’s solution, Donovan’s solution, and Asiatic pills for treatment of various illnesses such as psoriasis, asthma, and syphilis. Medicinal exposure is now basically limited to treatment of tropical diseases, such as African trypanosomiasis, and more recently to treat various hematologic malignancies.109 Arsenic in opium has been and is still used for medicinal and recreational purposes in India, and inorganic arsenic is still found in some traditional Chinese herbal preparations.110 Unfortunately, these substances can be easily purchased and are increasingly being used again as homeopathic remedies, resulting in modern day cases of what once was primarily a disease of historical interest.111,112
Knowledge of the medicinal benefits and poisoning potential of arsenic dates back to ancient times. Arsenic was introduced into the United States Pharmacopoeia in 1850, and before its use was discontinued around 1965, it was employed medicinally in the United States and Europe in the form of Fowler’s solution, Donovan’s solution, and Asiatic pills for treatment of various illnesses such as psoriasis, asthma, and syphilis. Medicinal exposure is now basically limited to treatment of tropical diseases, such as African trypanosomiasis, and more recently to treat various hematologic malignancies.109 Arsenic in opium has been and is still used for medicinal and recreational purposes in India, and inorganic arsenic is still found in some traditional Chinese herbal preparations.110 Unfortunately, these substances can be easily purchased and are increasingly being used again as homeopathic remedies, resulting in modern day cases of what once was primarily a disease of historical interest.111,112
![]() Arsenic exposure can occur in a variety of occupations, either by direct exposure to arsenic or through indirect exposure from contaminated water and landfills. In 1973, it was estimated that more than 1.5 million workers in the United States were potentially exposed to arsenic in the workplace.113 Occupations that carry risk of exposure include jobs in the mining, smelting, agricultural, computer microchip, forestry, electroplating, semiconductor, and glassmaking industries. Environmental exposure is often obscure and insidious, because there may be latent periods of up to 50 years before manifestations of chronic arsenicism appear. Arsenic is routinely found in soil, and it can accumulate in ground water and well water from these natural sources. It is becoming a public health concern in many areas, including Bangladesh, India, Taiwan, Japan, Mexico, Chile, Argentina, Canada, Pakistan, and the United States.114 Other routes of environmental exposure include some illegally produced alcoholic beverages and the burning of pressure-treated lumber that has been pretreated with chromated copper arsenate. In all forms of medicinal, occupational, and environmental exposures, longer duration of exposure and higher cumulative dose are associated with a higher risk for the development of ArKs.
Arsenic exposure can occur in a variety of occupations, either by direct exposure to arsenic or through indirect exposure from contaminated water and landfills. In 1973, it was estimated that more than 1.5 million workers in the United States were potentially exposed to arsenic in the workplace.113 Occupations that carry risk of exposure include jobs in the mining, smelting, agricultural, computer microchip, forestry, electroplating, semiconductor, and glassmaking industries. Environmental exposure is often obscure and insidious, because there may be latent periods of up to 50 years before manifestations of chronic arsenicism appear. Arsenic is routinely found in soil, and it can accumulate in ground water and well water from these natural sources. It is becoming a public health concern in many areas, including Bangladesh, India, Taiwan, Japan, Mexico, Chile, Argentina, Canada, Pakistan, and the United States.114 Other routes of environmental exposure include some illegally produced alcoholic beverages and the burning of pressure-treated lumber that has been pretreated with chromated copper arsenate. In all forms of medicinal, occupational, and environmental exposures, longer duration of exposure and higher cumulative dose are associated with a higher risk for the development of ArKs.
![]() The association between arsenic exposure and skin cancer was first noted by Paris in 1822.115 ArKs were first described on the palms and soles of individuals in the late 1800s. In 1898, Dubreuilh categorized ArKs as precancerous lesions.5 The relationship of arsenic ingestion to palmar and plantar ArKs and to skin cancer has been documented in several studies that examined wide-scale exposure to known sources of arsenic.116 In particular, chronic arsenicism and an increased risk of skin cancer have been attributed to drinking water that contains more than 0.6 mg of arsenic per liter.114 The US Environmental Protection Agency has set a maximum arsenic contaminant level at 50 μg/L.116 Ingestion of as little as 400 mL of Fowler’s solution can be associated with signs of chronic arsenicism.113 A compilation of the results of 12 reports that linked malignancy with the ingestion of inorganic arsenic found that skin cancer occurred in 70% and an internal malignancy in 6.3% of ingesters.117
The association between arsenic exposure and skin cancer was first noted by Paris in 1822.115 ArKs were first described on the palms and soles of individuals in the late 1800s. In 1898, Dubreuilh categorized ArKs as precancerous lesions.5 The relationship of arsenic ingestion to palmar and plantar ArKs and to skin cancer has been documented in several studies that examined wide-scale exposure to known sources of arsenic.116 In particular, chronic arsenicism and an increased risk of skin cancer have been attributed to drinking water that contains more than 0.6 mg of arsenic per liter.114 The US Environmental Protection Agency has set a maximum arsenic contaminant level at 50 μg/L.116 Ingestion of as little as 400 mL of Fowler’s solution can be associated with signs of chronic arsenicism.113 A compilation of the results of 12 reports that linked malignancy with the ingestion of inorganic arsenic found that skin cancer occurred in 70% and an internal malignancy in 6.3% of ingesters.117
![]() Arsenic is present as either organic or inorganic compounds as well as in three potential oxidative states: metalloid, trivalent, and tetravalent. Trivalent arsenicals are the most common and hazardous to humans. The toxicity of these compounds depends on the accumulation of arsenic in target tissues and its metabolism and elimination. Organic arsenicals are excreted rapidly. Trivalent inorganic arsenicals are the most acutely and chemically toxic compounds. Because arsenic is metabolized and detoxified in the liver via methylation, patients with preexisting liver disease may be at greater risk for arsenic-related toxicity.
Arsenic is present as either organic or inorganic compounds as well as in three potential oxidative states: metalloid, trivalent, and tetravalent. Trivalent arsenicals are the most common and hazardous to humans. The toxicity of these compounds depends on the accumulation of arsenic in target tissues and its metabolism and elimination. Organic arsenicals are excreted rapidly. Trivalent inorganic arsenicals are the most acutely and chemically toxic compounds. Because arsenic is metabolized and detoxified in the liver via methylation, patients with preexisting liver disease may be at greater risk for arsenic-related toxicity.
![]() The mechanisms of arsenic-induced keratoses and malignancy are not fully understood. Arsenic reacts with the sulfhydryl groups in certain tissue proteins and subsequently affects many different enzymes that are essential to cellular metabolism. Arsenic has been found to cause chromosomal mutations, chromosomal breaks, sister chromatid exchanges, and mutations in p53.118,119 A recent study demonstrated that polymorphisms in a nucleotide excision repair pathway gene, ERCC2, are associated with an increased susceptibility to arsenic-induced genotoxic effects and the formation of ArKs.120
The mechanisms of arsenic-induced keratoses and malignancy are not fully understood. Arsenic reacts with the sulfhydryl groups in certain tissue proteins and subsequently affects many different enzymes that are essential to cellular metabolism. Arsenic has been found to cause chromosomal mutations, chromosomal breaks, sister chromatid exchanges, and mutations in p53.118,119 A recent study demonstrated that polymorphisms in a nucleotide excision repair pathway gene, ERCC2, are associated with an increased susceptibility to arsenic-induced genotoxic effects and the formation of ArKs.120
![]() ArKs typically begin as pinpoint papules that are more easily felt than seen. They develop into small, 2–0-mm, punctate, yellow, keratotic papules most commonly seen on the palms and soles in areas of constant pressure or repeated trauma (eFig. 113-6.2A). They preferentially arise on the thenar and lateral borders of the hands, the sides of the fingers, and sometimes on the dorsal aspects of the fingers, overlying the joints. Although it is unusual, ArKs can be found on more widespread body areas such as the trunk, extremities, eyelids, and genitalia. ArKs may also present as slightly elevated, erythematous, scaly, or pigmented plaques. ArKs on the palms and soles are more likely to be seen in individuals with chronic arsenicism caused by medicinal exposure than in those with arsenicism due to occupational exposure. Also, individuals with arsenical-related cancer are more likely to have palmar and plantar ArKs. The mean latency period for the development of ArKs varies considerably from 9 to 30 years.121
ArKs typically begin as pinpoint papules that are more easily felt than seen. They develop into small, 2–0-mm, punctate, yellow, keratotic papules most commonly seen on the palms and soles in areas of constant pressure or repeated trauma (eFig. 113-6.2A). They preferentially arise on the thenar and lateral borders of the hands, the sides of the fingers, and sometimes on the dorsal aspects of the fingers, overlying the joints. Although it is unusual, ArKs can be found on more widespread body areas such as the trunk, extremities, eyelids, and genitalia. ArKs may also present as slightly elevated, erythematous, scaly, or pigmented plaques. ArKs on the palms and soles are more likely to be seen in individuals with chronic arsenicism caused by medicinal exposure than in those with arsenicism due to occupational exposure. Also, individuals with arsenical-related cancer are more likely to have palmar and plantar ArKs. The mean latency period for the development of ArKs varies considerably from 9 to 30 years.121
![]() Other cutaneous neoplasms associated with chronic arsenicism include BD or SCC in situ, SCC (see Chapter 114), and BCC (see Chapter 115). Mean latency periods for the development of BD and SCC can also be as long as 40 years.121 With arsenic as the carcinogen rather than UV radiation, these neoplasms, like ArKs, are distributed in more widespread, random, and nonphotodamaged areas. Arsenical BD often begins as a small flesh-colored to pink papule with a thick horny layer or crust. When this crust is removed, the underlying skin appears erythematous and oozing. Over time, unlike the relatively stable ArKs, these lesions increase in size, forming nodular and plaque-like lesions (see eFig. 113-6.2B) that often group together. Approximately one-third of patients have multiple lesions of BD.110
Other cutaneous neoplasms associated with chronic arsenicism include BD or SCC in situ, SCC (see Chapter 114), and BCC (see Chapter 115). Mean latency periods for the development of BD and SCC can also be as long as 40 years.121 With arsenic as the carcinogen rather than UV radiation, these neoplasms, like ArKs, are distributed in more widespread, random, and nonphotodamaged areas. Arsenical BD often begins as a small flesh-colored to pink papule with a thick horny layer or crust. When this crust is removed, the underlying skin appears erythematous and oozing. Over time, unlike the relatively stable ArKs, these lesions increase in size, forming nodular and plaque-like lesions (see eFig. 113-6.2B) that often group together. Approximately one-third of patients have multiple lesions of BD.110
![]() Arsenic-induced BCCs are also usually multiple, and the majority are of the superficial type, although small nodular BCCs are sometimes seen. These BCCs are found in a random, scattered distribution, primarily on the trunk and in hair-bearing regions. Clinically, the superficial BCCs are often indistinguishable from BD.
Arsenic-induced BCCs are also usually multiple, and the majority are of the superficial type, although small nodular BCCs are sometimes seen. These BCCs are found in a random, scattered distribution, primarily on the trunk and in hair-bearing regions. Clinically, the superficial BCCs are often indistinguishable from BD.
![]() Arsenic-related SCC can arise de novo or from malignant transformation of ArKs and BD lesions. In one study, 55% of SCCs arose from preexisting ArKs or BD lesions.121 Patients with SCC are more likely to have been exposed to arsenic earlier in life than are those without SCC.121 They also are more likely to have multiple lesions of BD and more numerous palmar and plantar ArKs. The majority of SCCs have been found on the distal extremities of the hands and feet, and it has been hypothesized that irritation and trauma in these areas may increase the risk of malignant transformation. Clinically, ArKs that show progression to SCC often present with pain, bleeding, fissuring, and later ulceration. They tend to gradually expand in diameter, forming a large erosion or ulceration, and they can, if neglected, reach sizes of up to 20 cm.
Arsenic-related SCC can arise de novo or from malignant transformation of ArKs and BD lesions. In one study, 55% of SCCs arose from preexisting ArKs or BD lesions.121 Patients with SCC are more likely to have been exposed to arsenic earlier in life than are those without SCC.121 They also are more likely to have multiple lesions of BD and more numerous palmar and plantar ArKs. The majority of SCCs have been found on the distal extremities of the hands and feet, and it has been hypothesized that irritation and trauma in these areas may increase the risk of malignant transformation. Clinically, ArKs that show progression to SCC often present with pain, bleeding, fissuring, and later ulceration. They tend to gradually expand in diameter, forming a large erosion or ulceration, and they can, if neglected, reach sizes of up to 20 cm.
![]() Other signs of chronic arsenicism include hyperpigmentation primarily affecting the nipples, axillae, groin, and other pressure points. Within these hyperpigmented patches are often small areas of guttate hypopigmentation, resembling “raindrops in the dust.” Diffuse alopecia of the scalp may be present. Longer term, patients may develop “blackfoot disease,” which is a peripheral vascular disorder affecting the lower extremities that eventually results in gangrene. Other systemic findings of chronic arsenicism include hematologic disorders, liver disease, sensorimotor neuropathy, hypertension, diabetes, and other gastrointestinal disturbances. A variety of internal malignancies have been associated with chronic arsenicism, with long latency periods of 20–50 years. The internal malignancies linked with chronic arsenicism include lung, urinary tract, and hepatic tumors, hepatic angiosarcoma, leukemia, and lymphoma.
Other signs of chronic arsenicism include hyperpigmentation primarily affecting the nipples, axillae, groin, and other pressure points. Within these hyperpigmented patches are often small areas of guttate hypopigmentation, resembling “raindrops in the dust.” Diffuse alopecia of the scalp may be present. Longer term, patients may develop “blackfoot disease,” which is a peripheral vascular disorder affecting the lower extremities that eventually results in gangrene. Other systemic findings of chronic arsenicism include hematologic disorders, liver disease, sensorimotor neuropathy, hypertension, diabetes, and other gastrointestinal disturbances. A variety of internal malignancies have been associated with chronic arsenicism, with long latency periods of 20–50 years. The internal malignancies linked with chronic arsenicism include lung, urinary tract, and hepatic tumors, hepatic angiosarcoma, leukemia, and lymphoma.
![]() The histopathologic features of ArKs are essentially the same as those of AKs. No reliable histopathologic criteria can distinguish between the two. Some cases of ArKs are characterized by marked vacuolation of the epithelial cells and keratin horn formation. Also, solar elastosis is usually absent, and a chronic dermal lymphocytic infiltrate is commonly seen. Likewise, arsenic-related BD, BCC, and SCC are histopathologically indistinguishable from their nonarsenic-induced counterparts.
The histopathologic features of ArKs are essentially the same as those of AKs. No reliable histopathologic criteria can distinguish between the two. Some cases of ArKs are characterized by marked vacuolation of the epithelial cells and keratin horn formation. Also, solar elastosis is usually absent, and a chronic dermal lymphocytic infiltrate is commonly seen. Likewise, arsenic-related BD, BCC, and SCC are histopathologically indistinguishable from their nonarsenic-induced counterparts.
![]() ArKs may be mistaken for other types of punctate keratoses, such as disseminated punctate keratoderma, Darier disease, corns, and verruca vulgaris. Disseminated punctate keratoderma usually appears earlier in life. It has also been reported that upon removal of the keratinous plugs in punctate keratoderma, small crater-like pits are left behind, whereas no pits are seen on removal of the keratin plugs in ArKs. Darier disease presents with characteristic lesions elsewhere. Corns are usually not so numerous and not so common on the hands. Warts often demonstrate evidence of thrombosed capillaries upon removal of the surface keratin.
ArKs may be mistaken for other types of punctate keratoses, such as disseminated punctate keratoderma, Darier disease, corns, and verruca vulgaris. Disseminated punctate keratoderma usually appears earlier in life. It has also been reported that upon removal of the keratinous plugs in punctate keratoderma, small crater-like pits are left behind, whereas no pits are seen on removal of the keratin plugs in ArKs. Darier disease presents with characteristic lesions elsewhere. Corns are usually not so numerous and not so common on the hands. Warts often demonstrate evidence of thrombosed capillaries upon removal of the surface keratin.
![]() A diagnosis of ArKs and chronic arsenicism should be considered when numerous characteristic keratoses are seen on the palms and soles or when multiple lesions of BD, SCC, or BCC are found on an individual, especially when these lesions are in nonsun-exposed regions of the body. In most patients with such neoplasms, palmar and plantar keratoses will also be present. Such patients should be questioned about previous occupations, living conditions, and environmental and medicinal exposures to elicit a history of potential arsenic exposure anywhere from 10 years to 40 years previously. Biopsy of any changing ArK, erythematous nodule, plaque, or ulcer on the body should be performed to ensure that BD, BCC, or progression to SCC is not present. In addition, laboratory tests to order include a complete blood count, renal and liver function tests, hair and nail analysis to measure arsenic concentration, and nerve conduction studies if a sensorimotor neuropathy is suspected.
A diagnosis of ArKs and chronic arsenicism should be considered when numerous characteristic keratoses are seen on the palms and soles or when multiple lesions of BD, SCC, or BCC are found on an individual, especially when these lesions are in nonsun-exposed regions of the body. In most patients with such neoplasms, palmar and plantar keratoses will also be present. Such patients should be questioned about previous occupations, living conditions, and environmental and medicinal exposures to elicit a history of potential arsenic exposure anywhere from 10 years to 40 years previously. Biopsy of any changing ArK, erythematous nodule, plaque, or ulcer on the body should be performed to ensure that BD, BCC, or progression to SCC is not present. In addition, laboratory tests to order include a complete blood count, renal and liver function tests, hair and nail analysis to measure arsenic concentration, and nerve conduction studies if a sensorimotor neuropathy is suspected.
ArKs and arsenical-induced BD tend to persist for many years, and progression to invasive SCC is believed to be relatively rare. However, invasive SCCs that arise in ArKs are more locally aggressive and have a greater chance of metastasis than SCCs arising in AKs.110 In lesions of arsenical-induced BD, locally invasive SCC has been seen histopathologically in up to 20% of cases. Once invasive SCC has occurred in BD, it is said that at least one-third will demonstrate evidence of metastasis unless adequate treatment is provided.122
Management of patients with chronic arsenicism and ArKs should include regularly scheduled total-body skin examinations and general physical examinations, possibly every 6 months.121 The exact incidence of internal malignancies associated with chronic arsenicism is unknown, so there is no standard protocol for the evaluation of potential internal malignancies. Exhaustive evaluations to detect such malignancies have not been recommended. Biannual detailed history taking and physical examination, yearly chest radiography, and selective testing when clinically indicated are probably reasonable recommendations.
Treatment of ArKs likewise is not standard and not mandatory, although treatment of these lesions is sometimes initiated to relieve the associated discomfort that some patients experience. Available treatment options include surgical excision, cryosurgery, curettage with or without electrosurgery, CO2 laser treatment, and topical chemotherapy with 5-FU, although 5-FU therapy is less successful in treating ArKs than in treating AKs.110 PDT has also been used to treat these lesions. Limited studies suggest that oral retinoids and keratolytics may be useful in treating ArKs.123,124 One case report of effective treatment of ArKs, BD, and BCC lesions in one patient with chronic arsenicism with topical 5% imiquimod cream has been reported.125
Thermal Keratoses
|
![]() Thermal keratoses (TKs) are keratotic lesions produced in the skin by exposure to infrared radiation. They have the potential to develop into SCC. Various sources of heat are implicated in causing these lesions, and several classic syndromes have been reported. Most notable are the kangri basket cancers in Kashmir, the kang cancers in China, the railway cancers in Britain, and the peat fire cancers in Ireland. The kangri is a pot that contains some type of fuel, such as charcoal, which is held against the thigh or lower abdomen as a source of warmth. In Kashmir, the malignancies would arise in the skin over the lower abdomen and thighs, where the baskets were in contact with the body. Kang are brick beds used primarily in the northeastern part of China that are usually heated by pipes connected to coal-burning stoves. The skin overlying the greater trochanter is where the cancers were most likely to develop. Heat was reported to be responsible for cancer of the skin overlying the shins of railway engine drivers in England. In Ireland, more than 150 women were reported to have lesions of erythema ab igne and SCC on the lower legs. These women all reported many years of sitting close to an open fire of burning peat to stay warm. Because peat is a less efficient fuel than coal, these women had to sit closer to the source to attain adequate warmth. The men did not experience the same cutaneous effects because they primarily wore thick, long trousers. In the United States, TKs, BD, and the development of SCCs have been noted in persons after long-term exposure to coal- and wood-burning stoves used as sources for heat and cooking.126 The incidence and prevalence of TKs are not known. Likewise, the pathogenesis of these lesions is not fully understood, and the percentage of TKs that will progress to invasive SCC is also unknown. Erythema ab igne, the precursor lesion, has been reported as a result of long-term exposure to laptop computers and electric heating pads.127,128
Thermal keratoses (TKs) are keratotic lesions produced in the skin by exposure to infrared radiation. They have the potential to develop into SCC. Various sources of heat are implicated in causing these lesions, and several classic syndromes have been reported. Most notable are the kangri basket cancers in Kashmir, the kang cancers in China, the railway cancers in Britain, and the peat fire cancers in Ireland. The kangri is a pot that contains some type of fuel, such as charcoal, which is held against the thigh or lower abdomen as a source of warmth. In Kashmir, the malignancies would arise in the skin over the lower abdomen and thighs, where the baskets were in contact with the body. Kang are brick beds used primarily in the northeastern part of China that are usually heated by pipes connected to coal-burning stoves. The skin overlying the greater trochanter is where the cancers were most likely to develop. Heat was reported to be responsible for cancer of the skin overlying the shins of railway engine drivers in England. In Ireland, more than 150 women were reported to have lesions of erythema ab igne and SCC on the lower legs. These women all reported many years of sitting close to an open fire of burning peat to stay warm. Because peat is a less efficient fuel than coal, these women had to sit closer to the source to attain adequate warmth. The men did not experience the same cutaneous effects because they primarily wore thick, long trousers. In the United States, TKs, BD, and the development of SCCs have been noted in persons after long-term exposure to coal- and wood-burning stoves used as sources for heat and cooking.126 The incidence and prevalence of TKs are not known. Likewise, the pathogenesis of these lesions is not fully understood, and the percentage of TKs that will progress to invasive SCC is also unknown. Erythema ab igne, the precursor lesion, has been reported as a result of long-term exposure to laptop computers and electric heating pads.127,128
![]() Prolonged exposure to infrared radiation and to sources of heat can clinically produce the characteristic lesion of erythema ab igne, which manifests as an erythematous to brownish, fixed, thick, reticulated patch on the skin overlying the area that has been habitually exposed to heat (see Fig. 75-21). It differs from livedo reticularis in that it is more brownish, usually nonblanchable, and fixed. The sites of predilection are the back and abdomen from exposure to hot water bottles and heating pads or blankets, and the lower legs from exposure to fires or heating units as sources of warmth and most recently the anterior thighs from chronic exposure to laptop computers.128 After many years of such exposure, TKs may appear within these patches of erythema ab igne. SCC in situ within such patches has also been reported, and as with solar-induced AKs, progression to invasive SCC from TKs can occur. Clinically, TKs present as hyperkeratotic papules and plaques.
Prolonged exposure to infrared radiation and to sources of heat can clinically produce the characteristic lesion of erythema ab igne, which manifests as an erythematous to brownish, fixed, thick, reticulated patch on the skin overlying the area that has been habitually exposed to heat (see Fig. 75-21). It differs from livedo reticularis in that it is more brownish, usually nonblanchable, and fixed. The sites of predilection are the back and abdomen from exposure to hot water bottles and heating pads or blankets, and the lower legs from exposure to fires or heating units as sources of warmth and most recently the anterior thighs from chronic exposure to laptop computers.128 After many years of such exposure, TKs may appear within these patches of erythema ab igne. SCC in situ within such patches has also been reported, and as with solar-induced AKs, progression to invasive SCC from TKs can occur. Clinically, TKs present as hyperkeratotic papules and plaques.
![]() The histopathologic features of TKs are similar to those of AKs, including alternating hyperkeratosis and parakeratosis, keratinocyte atypia, sparing of the appendageal units, and, interestingly, even dermal elastosis and vascular ectasia. In one study, biopsy specimens from erythema ab igne patches without apparent TKs showed evidence of keratinocyte atypia.129 Merkel cell carcinoma has also been reported to arise alongside SCC within a patch of erythema ab igne.130
The histopathologic features of TKs are similar to those of AKs, including alternating hyperkeratosis and parakeratosis, keratinocyte atypia, sparing of the appendageal units, and, interestingly, even dermal elastosis and vascular ectasia. In one study, biopsy specimens from erythema ab igne patches without apparent TKs showed evidence of keratinocyte atypia.129 Merkel cell carcinoma has also been reported to arise alongside SCC within a patch of erythema ab igne.130
![]() Biopsy should be performed on hyperkeratotic papules or plaques that develop within a long-standing patch of erythema ab igne to confirm the diagnosis and to rule out progression to SCC.
Biopsy should be performed on hyperkeratotic papules or plaques that develop within a long-standing patch of erythema ab igne to confirm the diagnosis and to rule out progression to SCC.
![]() There is a paucity of information in the literature regarding the risk of progression of TK to invasive SCC and the general prognosis of thermal SCC. Similarly, little has been written about the treatment of these lesions. Surgical excision, curettage with or without electrosurgery, cryosurgery, and possibly laser therapy appear to be reasonable therapeutic options. Patients with erythema ab igne should be counseled about the need to discontinue exposure to whatever heat source they have been in contact with, and they should be followed long term with physical examinations to identify early changes suggestive of TK or SCC.
There is a paucity of information in the literature regarding the risk of progression of TK to invasive SCC and the general prognosis of thermal SCC. Similarly, little has been written about the treatment of these lesions. Surgical excision, curettage with or without electrosurgery, cryosurgery, and possibly laser therapy appear to be reasonable therapeutic options. Patients with erythema ab igne should be counseled about the need to discontinue exposure to whatever heat source they have been in contact with, and they should be followed long term with physical examinations to identify early changes suggestive of TK or SCC.
Hydrocarbon Keratoses
|
![]() Hydrocarbon keratoses (HKs), also known as tar keratoses, pitch keratoses, and tar warts, are precancerous keratotic skin lesions that occur primarily in persons who are exposed occupationally to polycyclic aromatic hydrocarbons (PAHs). SCC and keratoacanthoma have also been linked to such exposures. PAHs are produced by the incomplete combustion and distillation of coal, natural gas, and oil shale. They are found in tar, fuel oils, lubricating oils, oil shale, and bitumen.115 Patients at risk for exposure to such compounds include workers in tar distillation and shale extraction, and workers exposed to creosotes, which are one of the main distillate fractions of coal tars and coal tar pitches.131
Hydrocarbon keratoses (HKs), also known as tar keratoses, pitch keratoses, and tar warts, are precancerous keratotic skin lesions that occur primarily in persons who are exposed occupationally to polycyclic aromatic hydrocarbons (PAHs). SCC and keratoacanthoma have also been linked to such exposures. PAHs are produced by the incomplete combustion and distillation of coal, natural gas, and oil shale. They are found in tar, fuel oils, lubricating oils, oil shale, and bitumen.115 Patients at risk for exposure to such compounds include workers in tar distillation and shale extraction, and workers exposed to creosotes, which are one of the main distillate fractions of coal tars and coal tar pitches.131
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree