Epidermal Stem Cells: Introduction
|
The cutaneous epithelium is a continually renewing tissue maintained in a dynamic equilibrium of proliferation in the basal layer and loss through terminal differentiation from the suprabasal layers. This process is orchestrated with great elegance by a hierarchy of stem cells, transient amplifying cells, and terminally differentiating cells. These populations of cells work together to maintain lifelong tissue function and to bring about tissue repair. This chapter focuses on the role of stem cells and their identification in the epidermis.
Concept of Stem Cells in Renewing Tissues
Proliferation in the cutaneous epithelium begins with the stem cells.1,2 Stem cells in this regard lack many characteristics of terminal differentiation, and have an intrinsically high proliferative potential relative to the other proliferating cells, but are generally capable of lifelong proliferation.3 Upon division, a stem cell produces off one daughter that remains a stem cell, and one daughter that goes on to produce a series of transit amplifying cells that serve to magnify or amplify the stem cell’s division resulting in the production of many differentiated cells with minimal input from the stem cell. This hierarchical system that usually involves decreasing proliferative potential is illustrated in Fig. 45-1. Stem cells typically interact with their surroundings in a supportive, protective niche.1
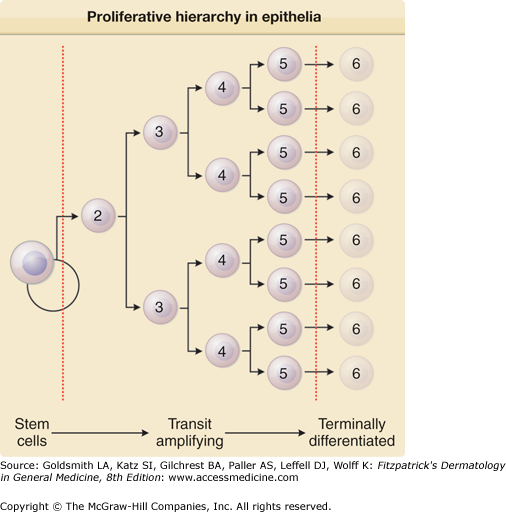
Figure 45-1
The proliferative hierarchy in epithelia: the stem cell concept. The ultimate progenitor cells are termed stem cells. They are slow cycling, long-lived, phenotypically undifferentiated, reside in specialized microenvironments, and constitute only a small percentage of the total epithelial cell population. Stem cell division produces transit amplifying or committed progenitor cells, which cycle rapidly and produce a clonal expansion of the offspring arising from an initial stem cell division. These cells eventually become the mature, terminally differentiated cells that constitute the bulk of a given epithelial population. Numbers indicate generation.
General Methods for Stem Cell Identification and Isolation
Stem cells may be studied by the presence or absence of proteins on their surface that distinguish them from other proliferative cells.2 Such proteins may be internal, or more desirably, proteins on the cell surface that render the cells selective by various methods such as by magnetic bead separation or by fluorescence activated cell sorting (FACS).2 Stem cells may also be studied by cellular kinetic characteristics such as in slowly cycling label-retaining4 cells or through their patterns of mitotic activity.5 Stem cells can sometimes be identified and isolated according to their special physical properties such as cell size or buoyant density,6 or in conjunction with other properties such as in the so-called “side population” cells7 that have active Abcg2 transporters. Candidate stem cells identified or isolated by any of these methods may then be characterized by functional tools such as in vitro colony forming assays,8–10 or in an in vitro or in vivo skin reconstitution assay.2
The hierarchical model for cell proliferation in the cutaneous epithelium implies some degree of cellular, genetic, or population asymmetry.3,11 The model of the stem cell hierarchy suggests a level of asymmetry that could be due to infrequent cell division or to chromosomal segregation.3 As the stem cell mechanism is thought to provide protection for the tissue as well as the cellular DNA, Cairns12,13 hypothesized that perhaps stem cells have a special mechanism for segregating their DNA and retaining an “immortal strand” at each division. Although there is some fairly convincing evidence that stem cells of the breast14 and intestinal epithelium15 may reserve an immortal DNA strand, recent investigation of the multipotential stem cells of the mouse hair follicles suggest that chromosome segregation does not occur.16,17 Moreover, the Tumbar laboratory developed a method to trace the proliferation history of hair follicle bulge keratinocytes and thus provided direct evidence in support of the infrequent division model for these particular stem cells.16
Cellular proliferation and terminal differentiation in the epidermis are usually thought to occur in columns. However, lateral migration is common during wound healing where a tongue of proliferating epithelium migrates over denuded dermis and reestablishes an epithelium complete with vertical proliferative units and terminal differentiation.18 In addition, Brash and colleagues have suggested that following irradiation of skin with ultraviolet light, clonal patches, each with its own p53 mutation, might reflect epidermal cell proliferation and differentiation beyond the confines of single proliferative units.19
Slowly cycling epidermal stem cells were first identified by of their cell kinetic behavior in the context of the epidermal proliferative units. Hence, Mackenzie identified mitotic basal keratinocytes beneath the edges of the hexagonal squames.20 In vertical cross sections of skin, Christophers21 had found that some basal cells in the shape of a hand mirror stained with a fluorescent dye characteristic of suprabasal cells. These studies were quantified by Potten in 197422 who called these units of structure epidermal proliferative units (EPUs). He focused on the central basal cells, noting that they were rarely mitotic and also rarely incorporated [3H]thymidine administered as a single pulse, and conjectured that the quiescent central cells might have stem cell activity whereas the peripheral cells may have transit amplifying cell activity. The next major advance in understanding the function of the EPU came with the identification of slowly cycling label-retaining cells in the center of the EPUs. Bickenbach4 and later Bickenbach and Mackenzie,23 Morris,24 and Potten25 found that administration of [3H]thymidine continuously for 3 days followed by a 4–8 week chase, could identify these central cells in light microscopic autoradiographs. Further characterization of the central stem cells and peripheral transit amplifying cells has been performed by a variety of in vivo and in vitro techniques.2 The presence of EPUs in some areas of thin human skin has also been noted, and may be as large as 2 millimeters in diameter.20 The EPU concept in skin is illustrated in Fig. 45-2.
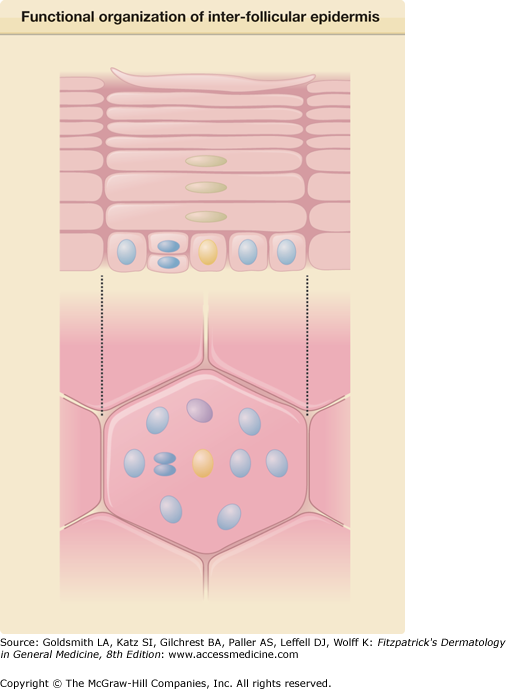
Figure 45-2
Functional organization of interfollicular epidermis: the epidermal proliferative unit (EPU) concept. Interfollicular epidermis of the skin of certain body sites is histologically organized into columns termed EPUs; each consists of approximately ten basal cells, including a single putative stem cell (yellow), its immediate transit-amplifying cell progeny (blue), and early-differentiating cells (purple). More differentiated keratinocytes (green) and then mature enucleated squames lie directly above them, in an ordered stack rising above the basal layer. EPUs represent functionally independent packets of self-renewing interfollicular epidermis that are ultimately dependent on a single putative stem cell for lifelong cell production. Constant self-renewal within the basal layer of skin compensates for the continual loss of differentiated squames from its surface. (Redrawn from Kaur P: Interfollicular epidermal stem cells: Identification, challenges, potential. J Invest Dermatol 126:1452, 2006.)
The distribution of stem cells within the epidermis has been a subject of debate. Hence, Lavker and Sun26 found that mitotic cells and cells that were rarely mitotic (putative stem cells) were located respectively in the upper and lower aspects of the rete ridges. These investigators postulated that the deeper cells were physically more protected than the superficial cells. In contrast, further studies have demonstrated that cells with stem cell characteristics in human epidermis can vary depending upon the site.27 Ghazizadeh and Taichman28 used retrovirally marked human epidermal keratinocytes followed by grafting onto athymic nude mice to visualize proliferative units in human skin. These studies found presumptive stem cells to be distributed throughout the epithelium.
Compared with the plethora of markers and selectable determinants for various hair follicle stem cells, few such markers have been identified in the epidermis. Notable exceptions are β-1 integrin,10 CD71 (the transferrin receptor),29 and LRIG1 (Fig. 45-3).30
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree