Diagnostic and Therapeutic Procedures
Overview
Because of the ready availability of the skin to examination, a number of diagnostic measures—many of which are noninvasive—can lead to a specific diagnosis.
Examples include potassium hydroxide (KOH) examination and fungal culture (see later discussions), Tzanck preparation (see Fig. 6.26), scabies preparation (see Fig. 20.18), Wood’s light examination (see Fig. 14.4), and patch testing (see Fig. 2.33).
Skin biopsies using punch, shave, snip, or excisional methods are relatively free of complications.
Therapeutic modalities such as cryosurgery (see later discussion), phototherapy (discussed in Chapter 3, “Psoriasis”) and advanced surgical procedures such as Mohs’ micrographic surgery (see later discussion) are but a few of the many available treatments for skin disorders.
 FUNGAL TESTS
FUNGAL TESTS
Potassium hydroxide test
Fungal culture
 SKIN BIOPSY
SKIN BIOPSY
Local anesthesia
Shave biopsy and shave removal
Scissor (snip) biopsy and snip excision
Punch biopsy
 SIMPLE ELLIPTICAL EXCISION
SIMPLE ELLIPTICAL EXCISION
Undermining technique
Wound closure
Suture material
Suturing
 COMEDO EXTRACTION SIMPLE PUNCH BIOPSY METHOD TO REMOVE CYSTS ELECTRODESICCATION AND CURETTAGE
COMEDO EXTRACTION SIMPLE PUNCH BIOPSY METHOD TO REMOVE CYSTS ELECTRODESICCATION AND CURETTAGE
Curettage
Electrodesiccation
 CRYOSURGERY
CRYOSURGERY
Cotton tip applicator technique
Cryospray technique
Postoperative course and wound care
 MOHS’ MICROGRAPhic SURGERY DRESSINGS AND WOUND MANAGEMENT
MOHS’ MICROGRAPhic SURGERY DRESSINGS AND WOUND MANAGEMENT
Potassium Hydroxide Test
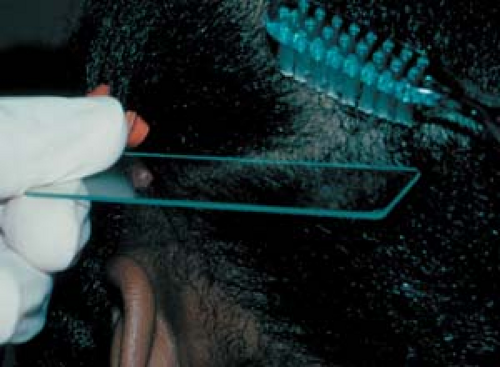 26.3 Potassium hydroxide examination. Collection of scale from the scalp of a child using a toothbrush. |
Basics
The KOH examination has the advantage of providing an immediate diagnosis of a superficial fungal infection, rather than having to wait weeks for the results of a fungal culture.
It is a simple, rapid method to detect fungal elements in skin, nails, and hair.
Technique
Collection of Specimen
Collection is optimal when no surface artifacts (e.g., topical medications) are present.
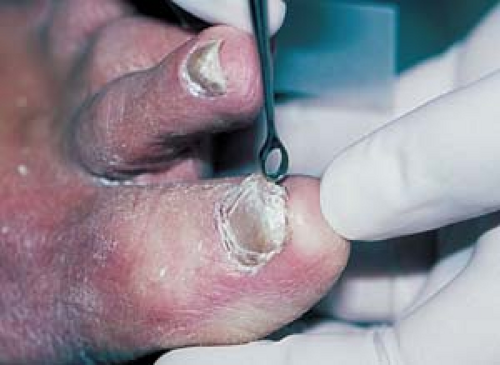
Nails
Trim the nail.
Use a no. 15 scalpel blade or a 1- to 2-mm curette (Fig. 26.2) under the nail surface to obtain scale.
Preparation
Use a KOH solution such as Swartz-Lamkins fungal stain or a KOH solution with dimethyl sulfoxide.
Gather a thin layer of scale or scale plus hair on a slide and cover it with a coverslip.
With an eyedropper, place a single drop of a KOH solution at the edge of the coverslip and allow it to spread under the coverslip by capillary action (Fig. 26.4).

26.4 Potassium hydroxide examination. A single drop of a KOH solution is placed at the edge of the coverslip.
Heat the undersurface of the slide gently with a lighter or a match until bubbling begins.
Blot excess KOH solution with tissue paper held at the edge of the coverslip.
Observation
Examine under low light intensity (condenser down).
Begin with a low-power scan to identify scale and possibly hyphae.
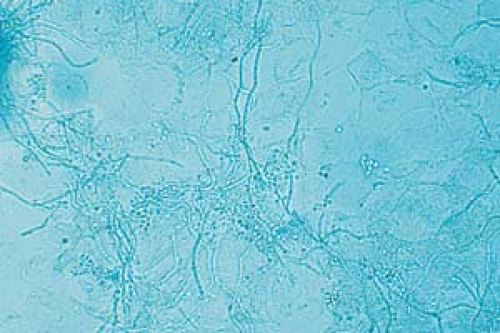
Become aware of artifacts that are easily confused with hyphae and spores, such as hairs, clothing fibers, keratinocyte cell borders, and air bubbles (Fig. 26.5).
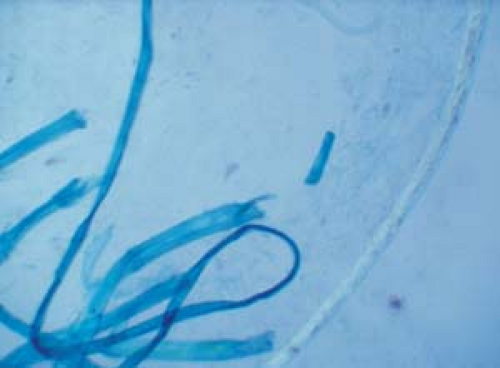 26.5 Potassium hydroxide examination. Artifacts. Note the clothing fibers on the left and the single hair shaft on the right. |
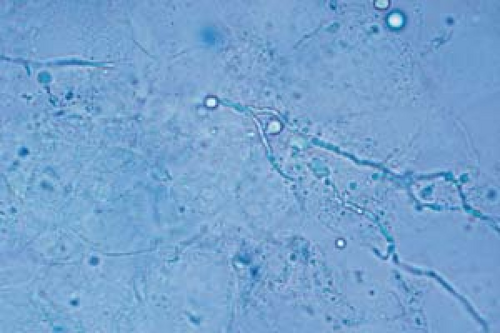 26.6 Potassium hydroxide examination. Dermatophyte. Note the wavy, branched hyphae with uniform widths coursing over cell borders. |
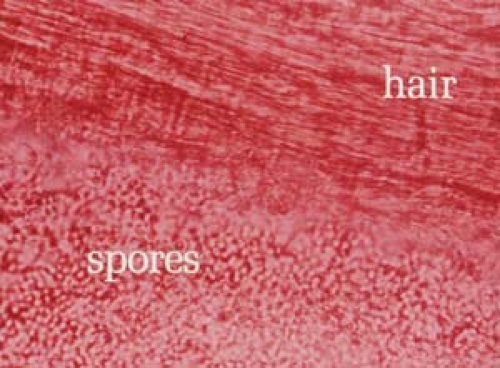
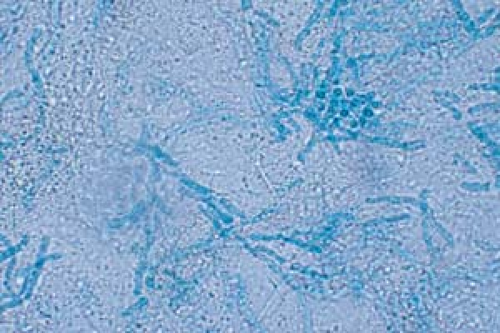 26.7 Potassium hydroxide examination. Tinea versicolor. Note the short, stubby hyphae (“spaghetti”) and the clusters of spores (“meatballs”). |
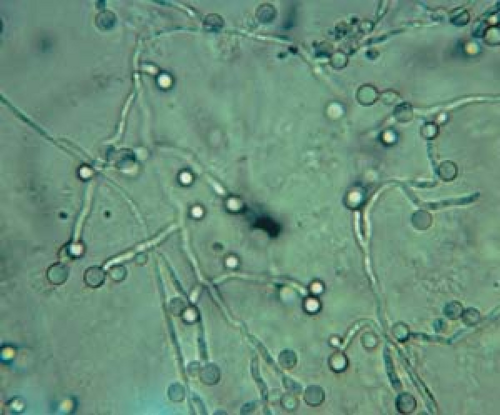 26.9 Potassium hydroxide examination. Candida. Pseudohyphae with budding spores (higher magnification). |
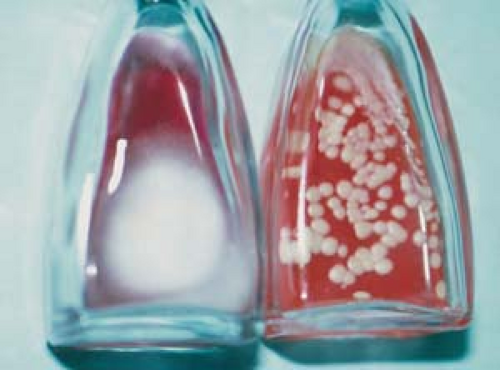
Culture
Place fungal cultures on Sabouraud’s agar or on Dermatophyte test medium and incubate for 1 to 4 weeks (Fig. 26.12).
Clinical Laboratory Improvement Act guidelines may require the practitioner to use outside laboratory facilities for performing fungal cultures.
Various skin biopsy techniques are available to the practitioner: shave biopsy, scissor or snip biopsy, punch biopsy, and excisional biopsy. The surgical tools and approaches vary according to size, shape, depth, and location of a lesion.
Obtaining the appropriate amount of tissue to provide adequate information about the disease is the most important factor to keep in mind when deciding on the proper biopsy technique.
Do not choose the biopsy specimen site indiscriminately.
Evaluate the site according to the clinical impression, the lesion’s location, the estimated depth of the pathologic process, the planned tissue studies, and the ensuing cosmetic result.
The choice of biopsy technique requires some knowledge of where the pathologic process is likely to be located.
Local Anesthesia
Methods to decrease pain caused by injections include the following.
Use a small, 30-gauge needle.
Add a buffer (sodium bicarbonate) to the lidocaine.
Inject very slowly.
Distract the patient by talking continuously.
Minimize the number of injection sites by reinjecting into areas that are already numb.
Skin Biopsy
Shave Biopsy and Shave Removal
Basics
This is used for the diagnosis and therapeutic removal of superficial (epidermal and upper dermal) skin lesions, such as melanocytic nevi, warts, seborrheic and solar keratoses, pyogenic granulomas, and skin tags, as well as other benign and malignant skin tumors.
It is used to obtain biopsy specimens to confirm skin disease before a more definitive surgical procedure (e.g., basal or squamous cell carcinoma).
It is very useful for flattening and diagnosing nevi, particularly in the facial area.
Advantages
It is fast and economical.
The technique is easy to learn.
Wound care is simple.
Cosmetic results are generally excellent.
It does not use sutures.
It is useful for difficult-to-reach sites (e.g., ear canal, orbit of the eye).
It is useful in areas of poor healing (e.g., the lower leg in elderly or diabetic patients).
Disadvantages
It is not indicated for lesions that extend into the subcutaneous layer.
It is not indicated when a full-thickness biopsy is necessary (e.g., inflammatory dermatoses).
It should not be performed on lesions suspected of being melanoma because of the difficulty in clinically determining the maximum thickness or extent of a lesion.
Technique
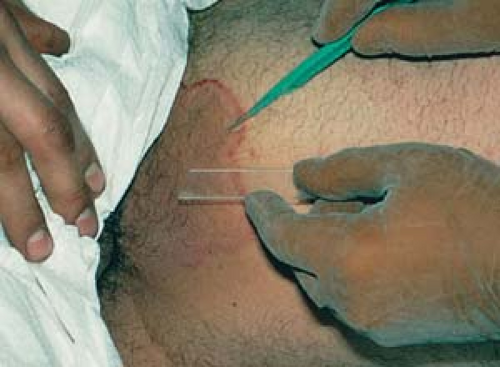
Anesthetize the area adjacent to the lesion with an injection into the superficial dermis with 1% plain lidocaine using a 30-gauge needle. If necessary, use epinephrine in a 1:100,000 or 1:200,000 dilution. Use lidocaine without epinephrine in finger and toe areas to avoid vascular compromise.
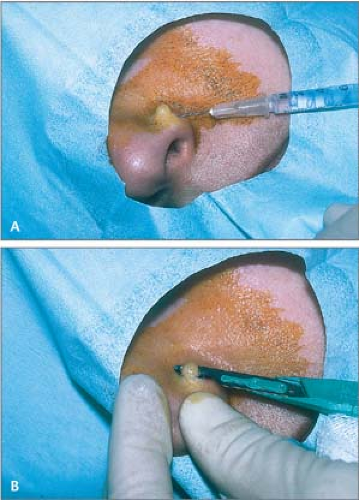
Administer local anesthesia with lidocaine to create a wheal and elevate the lesion above the surrounding skin (Fig. 26.13A).
Apply traction with the thumb and index finger of the free hand on either side of the lesion to stabilize it.
Place a no. 15 scalpel blade flat on the skin; use in a slight sawing motion with smooth strokes parallel to the skin surface and draw the middle of the blade through the lesion (Fig. 26.13B).
Release traction when the lesion becomes sufficiently free; use small forceps with teeth to hold and elevate the lesion to complete the “shave” and then to deliver it to a bottle of formalin.
Use electrocautery or further shaving to “feather” jagged edges.
Apply Monsel’s solution (ferric subsulfate) or aluminum chloride 35% with a cotton pledget (Q-Tip) to rapidly achieve hemostasis. Hemostasis is possible only if the field is wiped dry of blood.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree