Fig. 66.1
Blood glucose concentration in non-diabetic Lewis rats observed in sciatic nerve repair study, average age at the procedure – 10 weeks and 4 days: fasting – after 16 h of fasting, 9 weeks old; (A) before the procedure, around 9 weeks old; (B) within 3 days after the procedure, (C) at 6 weeks after the procedure (around 17 weeks old), (D) at 12 weeks after the procedure (around 23 weeks old); (1) indicates blood acquisition in the morning (8–9 A.M.), (2) indicates blood acquisition in the afternoon (4–5 P.M.)
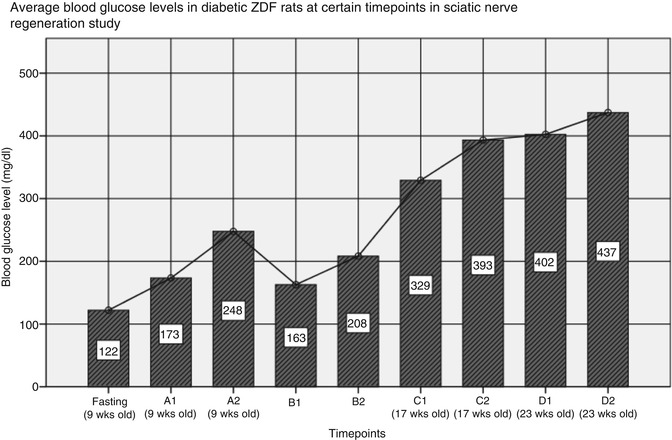
Fig. 66.2
Blood glucose concentration in diabetic ZDF rats observed in sciatic nerve repair study, average age at the procedure – 10 weeks and 5 days: fasting – after 16 h of fasting, 9 weeks old; (A) before the procedure, around 9 weeks old; (B) within 3 days after the procedure, (C) at 6 weeks after the procedure (around 17 weeks old), (D) at 12 weeks after the procedure (around 23 weeks old); (1) indicates blood acquisition in the morning (8–9 A.M.), (2) indicates blood acquisition in the afternoon (4–5 P.M.)
Blood Acquisition Method
A drop of blood for analysis can be obtained from tail capillary vessels. In this technique a person restrains rat with one hand using a plastic cone, with a tip of the cone cut off to allow the animal access to the fresh air. Tail needs to be warmed up by immersing it in warm water for few seconds. After tail is dried with a tissue, a small incision or puncture is made at the tail tip using a sterile lancet or a needle. “Milk-out” technique helps to obtain full droplet, that should be directly transferred onto a glucometer strip. Blood acquisition can be repeated daily from the same tail tip.
Organ-Specific Complications of Diabetes in Rats
Chronic hyperglycaemia or hyperinsulinaemia lead to a variety of complications. Some of the diabetic rat strains described above represent valuable models of complications of diabetes. Due to the slow development of disease Goto-Kakizaki rat was used in studies of ongoing diabetic complications: nephropathy [17], peripheral neuropathy [18] and retinal lesion [19]. Diabetic neuropathy in T1D was studied using Bio breeding BB/Wor rats [20]. Studies on chronic renal disease in T2D were conducted with use of OLETF rat model, allowing 50 weeks-long observation [21]. According to Cefalu and coauthors microvascular and cardiovascular complications are already present during the pre-diabetic metabolic syndrome initial phase, before the clinical onset of diabetes (i.e., in human increased risk for CVD 15 years before the diagnosis of DM) [9]. No animal model of diabetic complications fully reflects their nature seen in human. Therefore model selection should be always adjusted to the study design. Ideally experiments should be performed using various complementary animal models of diabetic complications to achieve rewarding results.
Nerve Regeneration in Diabetes-Induced Neuropathy
Peripheral neuropathy is one of the most commonly found complication of diabetes. Several rodent and non-rodent animal models were proposed to be used to study this topic. Pathogenesis of this particular complication is yet not fully understood, therefore no single experimental model of neuropathy reflecting human condition is known. Generally degeneration of all fiber types in affected nerve occurs in conjunction with chronic hyperglycaemia and hyperinsulinaemia. Clinical sensory lost, increased vibration and thermal perception thresholds, paresthesia, hyperalgesia and spontaneous pain are present as a result [22]. These symptoms are very subjective and their assessment is difficult in experimental studies using animals. Thus design of such study should be aimed at evaluation of objective results, like histopathology, direct observation of nerve lesion or regeneration, neuronal electrophysiology measurements or animal-independent clinical tests based on reflexes. Non-diabetic controls are needed for comparison.
In our study we used ZDF rats to evaluate sciatic nerve regeneration after injury (20 mm gap) and subsequent repair by either autologous nerve graft or a novel technique using autologous hollow epineural sheath conduit graft (experimental groups) (Fig. 66.3). A respective control groups of non-diabetic Lewis rats were treated according to the same protocol. Number of animals in every group was eight. Criteria of inclusion for ZDF rats were: glycaemia >200 mg/dl (twice) and glycemia after 16 h of fasting >110 mg/dl (overt diabetes). Animals were not treated with oral antidiabetics or exogenous insulin. Lewis rats were matched with ZDF rats for similar weight at the day of surgical procedure. Nerve regeneration was assessed 12 weeks after the operation. Relative results showed impaired regeneration in diabetic rats, when compared to the control groups. Clinical sensory and motor tests gave 22–29 % worse results in ZDF groups. Conduction velocity in regenerated nerve in diabetic animals was reduced by 4–9 % at week 12 after the procedure (assessed in somatosensory evoked potentials (SSEP) protocols) (unpublished data). Denervation atrophy in surgically reinervated muscles was significantly milder in non-diabetic rats (micromorphometric muscle fiber assessment), ending up with 53 % of control fiber cross-sectional area retained in Lewis vs 42 % in ZDF rats [23]. To the best of our knowledge this is the first study using experimental animal type 2 diabetes model in assessment of peripheral nerve regeneration. It is also the first application of epineural sheath conduit in nerve repair under diabetic conditions.