Abstract
Cutaneous neurophysiology encompasses specific itch mediators and nerve fibers that transmit itch peripherally and centrally. This chapter provides an overview of fundamental mechanisms of itch, from the skin to the brain. There are two subsets of pruritoceptive C neurons which respond to histamine versus cowhage and then activate distinct spinothalamic tract neurons. Peripheral itch mediators include histamine, proteases, and interleukin-31, while central itch mediators include opioids, gastrin-releasing peptide, and B-type natriuretic peptide. There is an overlap between chronic itch and chronic pain, including activation of multiple brain areas and associated neuromediators and receptors such as nerve growth factor and transient receptor potential (TRP) channels. Therapy with topical and systemic drugs that reduce itch sensitization by counteracting the responsible mediators represent promising treatment strategies.
Keywords
pruritus, primary sensory afferent neurons, C nerve fibers, pruritoceptive C neurons, nerve growth factor, neurotrophins, cowhage, histamine, opioids, substance P, gastrin-releasing peptide, B-type natriuretic peptide, transient receptor potential channels, interleukin-31, cathepsin S, MAS-related G protein coupled receptors, alloknesis, hyperknesis
- ▪
Two subsets of pruritoceptive C neurons that respond to histamine versus cowhage and then activate distinct spinothalamic tract neurons
- ▪
Peripheral itch mediators include histamine, proteases, and interleukin-31, while central itch mediators include opioids, gastrin-releasing peptide, and B-type natriuretic peptide
- ▪
There is an overlap between chronic itch and chronic pain, including activation of multiple brain areas and associated neuromediators and receptors, e.g. nerve growth factor, neurotrophin 4, transient receptor potential (TRP) channels
- ▪
Cross-talk between cutaneous nerve fibers and the stratum corneum is a possible mechanism for the pruritus associated with impaired barrier function (e.g. xerosis, atopic dermatitis)
- ▪
Therapies with topical and systemic drugs that reduce itch sensitization by counteracting the responsible mediators represent promising treatment strategies
Introduction
The skin is a sensory organ with a dense network of highly specialized afferent sensory nerves that convey sensations such as pain, itch, touch, temperature, vibration, and pressure ( Table 5.1 ); efferent autonomic nerve branches are also present. Neuropeptides such as substance P, calcitonin gene related peptide (CGRP), nerve growth factor (NGF), and other neurotrophins are secreted from nerve fibers, with multiple effects that include immune modulation. Itch (pruritus) is the dominant symptom of many cutaneous diseases. Almost all inflammatory skin disorders can result in pruritus, which patients often perceive as their most unendurable symptom. Pruritus can also occur in association with systemic disease (e.g. renal failure, cholestasis; see Ch. 6 ), psychiatric conditions (see Ch. 7 ), and damage to nerve fibers . Itch is a multidimensional phenomenon with sensory discriminative, cognitive, evaluative, and motivational components. In most instances, itch results from interactions that involve the brain–skin axis.
| PRIMARY AFFERENT NEURONS THAT INNERVATE THE SKIN | ||||
|---|---|---|---|---|
| Fiber | Diameter | Myelination | Conduction velocity | Responds to |
| A-beta (Aβ) | Large | + | >30 m/s | Light touch Moving stimuli |
| A-delta (Aδ) | Small | + | 2–30 m/s | Pain (nociceptors) Itch, cowhage-sensitive ‡ Thermal Mechanical |
| C | Small | − | <2 m/s | Pain (nociceptors) Itch * , histamine-sensitive † Itch * , cowhage-sensitive ‡ Thermal † Mechanical |
* ~5% of total C fibers transmit itch.
‡ Transmit pruritus accompanied by a burning sensation; also sensitive to mechanical stimuli.
† Separate C fibers carry both pruritogenic and thermal stimuli, but not mechanical stimuli.
Pruritus has many similarities to pain. Both are unpleasant sensory experiences that can impair quality of life in affected individuals. However, the behavioral reaction patterns differ – pain elicits a reflex withdrawal, whereas itch leads to a scratching response . Despite being an extremely common complaint and a sensation so rudimentary that almost every two- or four-footed creature experiences it, medical science is still struggling to understand the mechanisms of itch and how it can be inhibited.
The connection between itch and scratching is so close that in some languages the same word refers to both itch and scratch. Itch is restricted to the skin, tracheal mucous membrane, and several mucocutaneous junctions (e.g. conjunctivae). Interestingly, nerves in the deep reticular dermis and subcutaneous fat do not transmit itch.
Pruritus Pathways
Itch neurophysiology includes specific itch mediators and nerve fibers that transmit itch peripherally and centrally . While the concept of pruritus as a sensory modality entirely separate from pain was not generally appreciated until the mid-twentieth century, studies have clearly identified individual histaminergic and non-histaminergic C fibers that transmit itch . C fibers that transmit itch have exceptionally slow conduction velocities (0.3–1.0 m/second) and innervate unusually wide territories.
Histamine-sensitive C fibers are sensitive to heat as well as pruritogenic stimuli but not to mechanical stimuli; in contrast, the vast majority of C fibers are sensitive to mechanical and heat stimuli but have little or no response to histamine . The co-responsiveness of itch-transmitting C fibers to temperature explains aggravation of pruritus in a warm environment. However, the ineffectiveness of oral antihistamines for most types of pruritus suggests that other fibers have important roles in itch sensation .
Indeed, a distinct parallel pathway of non-histaminergic C fibers that transmit itch has been identified in the peripheral nervous system of humans and the spinothalamic tract of other primates . These fibers are activated by spicules of the tropical legume cowhage ( Mucuna pruriens ), which induces an intense sensation of itch when rubbed, inserted, or injected into the skin, without producing a histaminergic axon reflex . Cowhage spicules induce itch via release of the protease mucunain, which activates proteinase-activated receptor (PAR)-2 and PAR-4 . Cowhage-sensitive fibers transmit a burning sensation together with itch and are also sensitive to mechanical and other stimuli. PAR-2 receptors have a major role in mediating itch in patients with atopic dermatitis (see Ch. 12 ). The non-histaminergic, polymodal C fibers stimulated by mucunain may also have clinical relevance in chronic itch. In addition to C fibers, A-delta fibers contribute to cowhage-evoked itch with a more rapid onset .
Other non-histaminergic C fibers respond to β-alanine and activate distinct populations of primate primary sensory neurons . Intradermal injection of β-alanine elicits itch but not a wheal and flare response. MAS-related G protein-coupled receptor D (MrgprD) is a β-alanine receptor that is exclusively expressed by these C fibers. In mice, toll-like receptor 7 (TLR7) is expressed in C fibers and thought to have a role in itch (especially that elicited by non-histaminergic pruritogens) but not in pain sensations; however, its role in human itch is questionable .
A number of other neuromediators and receptors have been identified in animal models, but their role in itch pathophysiology in humans remains to be determined . Gastrin-releasing peptide receptor (GRPR)-positive neurons in the spinal cord of mice transmit itch, but not pain, via VGLUT2-mediated signaling . Overexpression of gastrin-releasing peptide in cutaneous nerve fibers and GRPR in the spinal cord has also been observed in mice and primates with chronic itch . B-type natriuretic peptide is an itch-selective neuropeptide found in dorsal root ganglia that is expressed at higher levels in mice than in rats and humans . Preprotachykinin A, a substance P precursor, is also expressed by a distinct population of murine dorsal horn neurons that respond to pruritic and noxious stimuli .
The perceived sensation of pruritus can vary greatly in quality . Patients may describe burning, pricking, “insects crawling” on the skin, or even a tickle, but the neurophysiologic and psychologic correlates of these qualitative differences have not yet been elucidated. However, information obtained from itch questionnaires has enabled a better understanding of the different characteristics of itch and its features in various skin diseases .
Central Pathways to Higher Nervous System Centers
In the spinal cord, pruriceptive C fibers synapse with secondary sensory neurons in the gray matter of the dorsal horn ( Fig. 5.1 ). These neurons then cross over and ascend in the lateral spinothalamic tract to the thalamus. Studies using in vivo extracellular recordings from cats identified a subclass of lamina I spinothalamic tract neurons that are excited by iontophoretically administered histamine . Neurophysiology experiments in primates then found that cowhage-induced itch stimulates a distinct population of spinothalamic tract neurons that are not involved in the transmission of histamine-mediated itch . Studies in human subjects utilizing functional MRI or positron emission tomography (PET) have shown that histamine and cowhage activate different brain areas, providing insight into the supraspinal processing of itch and the corresponding scratch response .
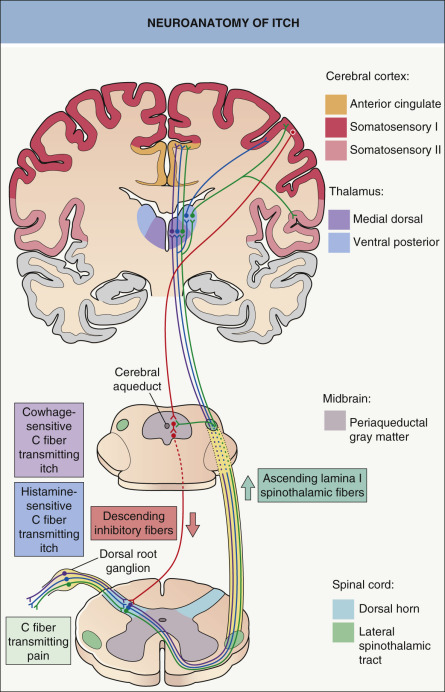
In healthy individuals, induction of itch by histamine or cowhage can elicit activation of the anterior and posterior cingulate cortex, precuneus, somatosensory areas I and II, supramarginal gyrus, inferior parietal lobe, and insula-claustrum complex. In addition to most of the previous areas, cowhage evokes more extensive activation of the insular cortex, claustrum, basal ganglia, putamen, and thalamic nuclei on the contralateral side of stimuli . In one study, atopic dermatitis patients exhibited significantly more activation of the posterior cingulate cortex and precuneus, a cortical area involved in integrative tasks such as visuospatial imagery, episodic memory retrieval and self-awareness, than did healthy controls . This highlights the emotional and affective processing of itch experience in atopic individuals, in whom the degree of brain activation correlated with itch intensity and the severity of the atopic disease .
In healthy subjects, scratching has been found to inhibit the activation of the cingulate cortex and to activate the prefrontal cortex and cerebellum. It is possible that the cerebellum plays a role in coordination of the itch–scratch cycle . Scratching also inhibits histamine-evoked activity of spinothalamic neurons in primates, but not spontaneous activity or that stimulated by pain . Self-scratching also activates areas of the brain involved in reward processing, including the striatum and substantia nigra. While these responses correlate with the pleasure of scratching, activation of other areas is associated with itch relief .
Activation of multiple brain areas argues against a single “itch center”, emphasizing the multidimensionality of the itch sensation. Pain demonstrates a similar pattern of brain activation involving many of the same cortical regions (see Fig. 5.1 ).
Seeing other people scratch can induce sensations of itch and an urge to scratch . Psychophysical studies have shown that the perception of itch intensifies in atopic dermatitis patients when they are exposed to visual cues of itch . Recent studies utilizing functional MRI showed that “contagious itch” activates many of the neural regions linked to the physical perception of itch, including anterior insular, primary somatosensory, prefrontal, and premotor cortices as well as the striatum . However, more studies are needed to verify the hypothesis that itch “contagion” involves processing in associative cortical networks such as “mirror neurons” of the prefrontal cortex.
Pruritus Receptor Units
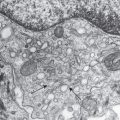
Removal of the epidermis abolishes the perception of itch, suggesting that putative pruritus receptor units are located predominantly within this layer. Light microscopic and ultrastructural studies of human skin have shown the existence of intraepidermal nerve fibers with “free” non-specialized nerve endings extending to the stratum granulosum . A subclass of C fibers in the epidermis that express Mrgprs, in particular MrgprX1, are involved in chloroquine-induced pruritus (see Fig. 5.2F ) . Cathepsin S, an endogenous protease, can cleave and thereby activate both MrgprC11 in mice, which leads to scratching behavior, and MrgprX2 in humans ; this is in addition to its ability to activate PAR-2/4 receptors ( Fig. 5.2 ). In mice, neurons that express MrgprA3 relay itch-specific information to the spinal cord .
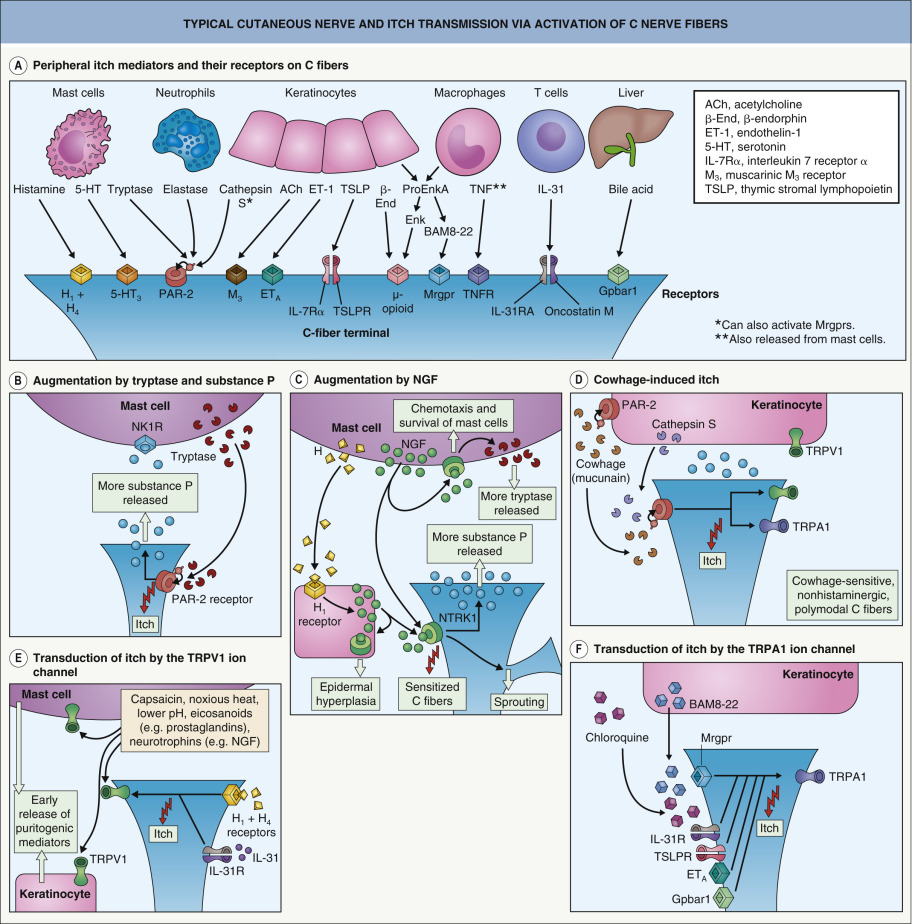
Keratinocytes express a variety of neural mediators and receptors involved in the sensation of itch ( Table 5.2 ). These include opioids, proteases, substance P, NGF, and neurotrophin 4 as well as their respective receptors, including µ- and κ-opioid receptors, PAR-2, neurotrophic tyrosine kinase receptor type 1 (NTRK1 [TRKA]), and transient receptor potential vanilloid ion channels (particularly TRPV1 and TRPV3). Keratinocytes also have ATP-gated ion channels and adenosine receptor ligands similar to those observed in C fibers involved in pain transmission. These structural similarities to nerve fibers suggest that keratinocytes may be involved in the transduction and generation of itch. PAR-2 receptors are thought to be involved in the itch of atopic dermatitis and mediate cowhage-induced itch (see above). Cathepsin S also induces itch via PAR-2/4 receptors and as mentioned previously, Mrgprs .
| MAJOR MEDIATORS OF PRURITUS: RELATIVE POTENCIES WITH REGARD TO PRURITUS AND PAIN | ||
|---|---|---|
| Mediator | Pruritus | Pain |
| Primary mediators | ||
| Histamine | +++ * | + * |
| Tryptase (protease) | +++ | + |
| Cathepsin S (protease) | +++ | ? |
| Interleukin-31 | +++ | ? |
| Secondary mediators | ||
| Prostaglandin E 1,2 | + † | + |
| Substance P ‡ | + | + |
| µ-Opioid receptor agonists | ++ | − |
| Nerve growth factor (NGF) | + | ++ |
| Interleukin-2 | +++ | +/− |
* Superficial (intraepidermal) injection of histamine causes pruritus; deep dermal injection causes pain.
† Lowers threshold to pruritus induced by other mediators.
Mediators of Pruritus
Various mediators that act centrally and/or peripherally are involved in pruritus, including histamine, proteases, substance P, opiates, NGF, and prostaglandins. In inflammatory skin diseases, proinflammatory mediators produce pruritus and other signs of inflammation, in particular erythema due to vasodilation and edema from increased vascular permeability. The relative potencies of the major mediators with regard to these responses (including pruritus) are listed in Table 5.2 . Some of these mediators cause pruritus indirectly by evoking release of histamine and tryptase from mast cells or by potentiating the actions of mediators such as prostaglandins E 1 and E 2 .
Histamine
Histamine is the archetypal mediator of signs and symptoms of inflammation, including pruritus. In the skin, histamine is contained primarily within the granules of dermal mast cells. Histamine can be released from mast cells upon activation of a range of receptors, including the high-affinity IgE receptor (FcεRI), the KIT receptor for stem cell factor, and receptors for neuropeptides (e.g. substance P, NGF) and complement C5a. In IgE-mediated acute urticaria, histamine is released when a specific antigen/allergen cross-links adjacent receptor-bound specific IgE antibodies. In autoimmune chronic urticaria, similar cross-linking occurs via functional circulating IgG that react with epitopes expressed on the α-chain of adjacent FcεRIs or less commonly anti-IgE autoantibodies (see Fig. 18.3 ). Histologically, dermal mast cells and unmyelinated neurons are closely juxtaposed (see Fig. 5.2B, C, E ), raising the possibility of a close (“synapsis-like”) functional relationship between the immune and nervous systems.
Histamine’s pruritic action can be potentiated by prostaglandin E 1 and E 2 . Evidence for histamine as the main mediator of pruritus is limited to a few skin diseases, including acute and chronic urticaria and mastocytosis (e.g. urticaria pigmentosa). H 1 antihistamines are usually effective in these disorders.
Recognition of the histamine H 4 receptor has expanded our understanding of the physiologic actions of histamine. The H 4 receptor is expressed by neurons and bone marrow-derived cells such as eosinophils, mast cells, dendritic cells, monocytes, and CD8 + T cells. It mediates chemotaxis in the latter group and is thought to have a role in the inflammation and pruritus of atopic dermatitis. H 4 antagonists are under development and have been shown to alleviate experimental pruritus. In animal models of itch, the effects of H 4 antagonists are synergistic with those of centrally acting H 1 antihistamines (e.g. diphenhydramine) .
Gastrin-Releasing Peptide
As noted above, itch-specific GRPR-positive neurons in the dorsal horn of the spinal cord have been identified in mice . Although named for its role in regulating gastrointestinal functions, the GRP ligand of GRPRs is widely expressed in the CNS. Whether peripheral sensory neurons express GRP is still debated .
B-Type Natriuretic Peptide
B-type natriuretic peptide (BNP; natriuretic polypeptide B) is a 32-amino acid polypeptide that is secreted by the cardiac ventricles to regulate blood pressure and fluid balance. BNP is also expressed by a subset of C fibers and may function in itch transmission , likely driving an itch circuit that includes GRPR-expressing neurons in the spinal cord.
Proteases
Human dermal mast cells produce two proteases, tryptase and chymase . Tryptase released by activated mast cells cleaves PAR-2, a G protein-coupled receptor present on C-fiber terminals (see Fig. 5.2B, D ); this exposes a tethered ligand domain and thereby “self-activates” PAR-2, leading to itch transmission. PAR-2 activation results in local release of neuropeptides, including substance P and calcitonin gene-related peptide, which induce neurogenic inflammation . Kallikrein and cathepsins in the skin can also activate PAR-2 in a similar manner. In addition, cleavage of murine MrgprC11 or human MrgprX2 by cathepsin S activates these receptors and evokes itch (see above).
Tryptase levels are elevated fourfold in non-lesional forearm skin of atopic dermatitis patients , while expression of PAR-2 is significantly increased in the epidermis and cutaneous nerve fibers of eczematous lesions and to a lesser degree in non-lesional skin. Proteases such as cathepsin B can also be found in common allergens (e.g. grass pollen, house dust mites) , and Staphylococcus aureus can induce secretion of proteases; both of these exogenous factors are known to aggravate atopic dermatitis and itch.
Transgenic mice overexpressing a serine protease exhibit severe itch and scratching ( Table 5.3 ). In Netherton syndrome, serine protease inhibitor deficiency leads to excess epidermal protease activity, resulting in pruritus and atopic manifestations (see Ch. 57 ). These observations suggest that interactions between proteases and receptors on C fibers play important roles in itch and cutaneous inflammation.
| MURINE MODELS OF ITCH | ||
|---|---|---|
| Model | Clinical and laboratory features | Pathogenesis/comments |
| Transgenic mice overexpressing PAR-2 | Epidermal hyperplasia and scaling as well as pruritus and scratching | |
| Transgenic mice overexpressing a serine protease | Pruritus and scratching | |
| TRPV1-deficient mice and Pirt-deficient mice | Lack of response to pruritogens | Pirt normally plays an important role in sensing itch (histaminergic and non-histaminergic) as well as pain via TRPV1 channels (and also TRPV1-independent itch) |
| Mrgpr-deficient mice and TRPA1-deficient mice | Lack of scratching response to chloroquine and BAM8-22, but preserved response to histamine | Mrgprs are normally activated by chloroquine and BAM8-22, so the lack of response in TRPA1-deficient mice implicates this receptor in downstream signaling |
| GRPR-deficient mice | Lack scratching response to pruritogens, but preserved response to painful stimuli | GRPR is expressed in the dorsal spinal cord |
| Bhlhb5-deficient mice | Enhanced scratching response to pruritogens | Selective loss of inhibitory interneurons in the dorsal horn that regulate pruritus |
| µ-Opioid receptor-deficient mice | Thinner epidermis, higher density of epidermal nerve endings, and less scratching after induction of dry skin | |
| Transgenic mice overexpressing interleukin-31 | Pruritus, scratching, excoriations and alopecia | |
Opioid Peptides
Central pruritus, which involves pruritic mediators within the CNS, can occur in both cutaneous and systemic diseases. Endogenous opiates modify the perception of pruritus via central and peripheral opioid receptors, and generalized pruritus may be induced by an imbalance between the µ- and κ-opioid systems. Activation of µ- and κ-opioid receptors stimulates and inhibits itch perception, respectively . κ-opioid receptor agonists can act within the skin, spinal cord (e.g. interneurons), and brain to reduce itch .
Morphine as well as other exogenous and endogenous µ-opioid receptor agonists may cause generalized pruritus . Morphine also produces local pruritus and erythema when injected intradermally; this response is only partially inhibited by the µ-opioid receptor antagonist naloxone but is substantially inhibited by topical pretreatment with the H 1 antihistamine doxepin . In contrast to morphine, the highly potent µ-opioid agonist fentanyl does not induce mast cell degranulation, even when applied in high doses. Therefore, two possible mechanisms for opioid-induced itch are: (1) degranulation of cutaneous mast cells ; and (2) activation of µ-opioid receptors with direct central and peripheral pruritogenic effects . Specifically, morphine-induced itch can result from activation of a heterodimeric µ-opioid and gastrin-releasing peptide receptor; inhibition of the latter receptor component blocked opioid-related itch but not analgesia, an observation that may have therapeutic relevance .
Nociceptin, the endogenous peptide ligand for the o pioid r eceptor- l ike 1 (ORL1) receptor, has also been implicated in cutaneous inflammation, pain, and pruritus . In a murine model, nociceptin interaction with ORL1 receptors on keratinocytes led to production of leukotriene B 4 , which induced scratching that was inhibited by systemic administration of naloxone .
Substance P
Substance P, a neuropeptide with a widespread distribution in peripheral nerves and the CNS, intensifies itch perception. Levels of substance P in the serum of patients with atopic dermatitis are elevated and correlate with disease severity . Intradermal injection of substance P provokes itch as well as elements of neurogenic inflammation such as erythema and the wheal-and-flare reaction. Substance P is synthesized in the cell bodies of C neurons, transported towards the peripheral nerve terminals, and released by antidromic depolarization to cause vasodilation and increased vascular permeability.
Although endogenously released substance P does not degranulate mast cells in healthy human skin or cause any sensation at physiologic concentrations , direct communication between nerve fibers and mast cells via substance P has been verified . High concentrations of substance P can cause immediate mast cell degranulation, whereas low concentrations specifically activate neurokinin-1 (NK-1) receptors on mast cells, leading to sensitization of these cells and increased production of tumor necrosis factor (TNF; see Fig. 5.2B ) . In turn, TNF sensitizes nociceptive nerve endings, producing a self-amplifying loop between neurons and mast cells. Substance P also plays an important role in spinal itch transmission .
Neurotrophins
Neurotrophins are factors that regulate the growth and function of nerve cells. Members of this family include the prototypic nerve growth factor (NGF) as well as brain-derived neurotrophic factor (BDNF) and neurotrophins 3, 4, and 5 . Increased levels of epidermal NGF correlate with proliferation of terminal cutaneous nerves and upregulated expression of neuropeptides such as substance P . NGF can also induce sprouting of nerve fibers, sensitization of nerve endings, and axonal transport in dorsal root ganglia cells (see Fig. 5.2C ) .
Keratinocytes express high levels of NGF, which is not only required for survival and regeneration of sensory neurons but also controls the responsiveness of such neurons to external stimuli . Patients with atopic dermatitis and psoriasis have increased expression of NGF in cutaneous mast cells, keratinocytes, and fibroblasts . NGF is thought to act as a signaling molecule between mast cells and keratinocytes in allergic skin diseases. Mast cell-derived histamine induces keratinocytes to increase NGF production, and the latter may promote infiltration of mast cells into inflamed skin . Upregulation of other neurotrophins, such as neurotrophin 4, has also been observed in keratinocytes from atopic dermatitis patients .
Prostanoids
In the skin, prostaglandins enhance histamine-induced itch . Prostaglandins are the products of the transformation of the essential fatty acid arachidonic acid by cyclooxygenase-1 (COX-1) or cyclooxygenase-2 (COX-2). When injected into the dermis, prostaglandin E 1 (PGE 1 ) is not itself pruritogenic but enhances pruritus due to histamine subsequently injected into the same site . It appears that only itch-mediating neurons that display lasting activation following exposure to histamine are excited by PGE 2 , and mechanosensitive fibers are unresponsive to both histamine and PGE 2 .
PGE 2 has also been shown to have a direct, low-level pruritogenic effect in both atopic dermatitis patients and unaffected individuals without inducing protein extravasation. This suggests that prostanoids’ peripheral action is not solely via histamine and that prostanoids may potentiate pruritus via other effects on nerve fibers.
Although oral administration of aspirin, a cyclooxygenase inhibitor, does not generally ameliorate pruritus , topical application of aspirin may reduce chronic localized itch . The role of other eicosanoids, including leukotrienes and 12-hydroxyeicosatetraenoic acid (12-HETE), in the pathogenesis of pruritus is unclear. In mice, leukotriene B 4 can provoke scratching and may be involved in skin disease-related itch .
Mediators That Activate Transient Receptor Potential Receptors
Neuromediators that activate ion channels belonging to the t ransient r eceptor p otential (TRP) family are also involved in the sensation of itch. TRP vanilloid 1 (TRPV1) is located on C fibers, dermal mast cells, dendritic cells, and keratinocytes (see Fig. 5.2E ) . This receptor is activated by capsaicin, endogenous substances such as cannabinoids (e.g. anandamide), prostaglandins and various neurotrophins, as well as by acidosis and temperatures >43°C (109°F); as a result, it can mediate heat pain. Mechano-insensitive pruritoceptive C-nerve pathways can be activated by capsaicin, indicating that there is expression of TRPV1. In addition, the experimental induction of histamine-mediated itch requires cooperation of TRPV1 ion channels . Stimulation of TRPV1-positive nerve fibers also leads to the release of multiple pruritoceptive mediators such as interleukins (ILs) and neuropeptides.
TRPV3 is a thermosensor of warmth (>33°C, 91°F) that is expressed in keratinocytes and dorsal root ganglion neurons in humans. In mice, a gain-of-function missense mutation in TRPV3 results in chronic itch, scratching, and an atopic-like dermatitis . In contrast, TRP melastatin 8 (TRPM8) on C nerve fibers functions as a thermosensor of cool temperatures (<28°C, 82°F) and is activated by menthol and icilin, which provide a “cooling” sensation that may relieve itch.
TRP ankyrin 1 (TRPA1), a polymodal nociceptor, functions as a downstream mediator of histamine-independent itch stimulated by Mrgprs present in a subset of epidermal C fibers . TRPA1 can be activated directly by menthol as well as via pruriceptors such as the heterodimeric receptor for thymic stromal lymphopoietin (TSLP), a cytokine released by keratinocytes that plays a role in the pruritus of atopic dermatitis (see Fig. 5.2F ).
Other Peripheral Mediators of Itch
Other neurotransmitters
Intradermal injection of acetylcholine, an important neurotransmitter in the autonomic nervous system, typically induces pain; however, in patients with chronic itch, it induces itch. In murine models of itch, activation of the muscarinic receptor 3 triggers pruritus . Norepinephrine, a catecholamine neurotransmitter, exerts tonic inhibition of itch signaling in the spinal cord . Currently there are no data regarding the role of epinephrine or dopamine in itch transmission.
Peptidases
Mast cells express both neuropeptides and neuropeptide-degrading peptidases including angiotensin-converting enzyme (ACE) and neural endopeptidases. Medications such as ACE inhibitors, which can induce pruritus without a rash, may induce itch by inhibiting the activity of these degrading enzymes. Likewise, the neural peptidase endothelin-converting enzyme 1 (ECE-1) negatively regulates endothelin 1-induced itch, which is largely histamine-independent .
Other mediators with potential roles in itch
Serotonin has been implicated as an inducer of itch in murine models; however, in humans it is a very mild pruritogen. Nitric oxide is another factor that may induce itch via neurogenic inflammation . The bovine adrenal medulla 8-22 (BAM8-22) peptide, a proteolytically cleaved product of proenkephalin A, is a potent activator of Mrgprs and can stimulate itch, usually accompanied by a stinging or burning sensation (see Fig. 5.2F ) . Chloroquine can also induce itch by activating Mrgprs, which are expressed by a subset of C fibers in the epidermis (see above).
Immune Cells as Itch Mediators and Modulators
Interactions between the nervous and immune systems in the skin have important roles in itch induction . Neuropeptides such as substance P, CGRP and vasointestinal peptide, which are released by cutaneous sensory nerves, can activate transcription factors and regulate the expression of adhesion molecules and proinflammatory cytokines, thereby modulating immune and inflammatory responses . These neuropeptides also influence cellular proliferation and differentiation, tissue repair, and antigen presentation involving keratinocytes, mast cells, dermal microvascular endothelial cells, and Langerhans cells. This interaction is bidirectional, as cytokines and chemokines are also able to regulate primary nerve afferents via receptor activation.
IL-2 is produced by activated T lymphocytes and causes pruritus when injected intradermally . High-dose IL-2 administered intravenously to patients with cancer (including stage IV melanoma) causes intense generalized pruritus. Moreover, treatment with topical calcineurin inhibitors, which block the production of IL-2, can result in decreased itch. Neither antihistamines nor NSAIDs reduce IL-2-induced pruritus, and whether the latter is directly receptor-mediated or an indirect effect via mast cells or endothelial cells remains to be determined.
IL-31 is produced by T helper 2 (Th2) cells and belongs to the IL-6 family. It induces pruritus by modulating the function of sensory neurons, with the itch developing after a mean delay of ~2 hours . IL-31 may exert its pruritogenic effect via activation of the IL-31 receptor (IL-31R) on keratinocytes, which could subsequently stimulate C fibers in the skin. The IL-31R is a heterodimer composed of the oncostatin M receptor (OSMR) β protein plus the IL-31 receptor A and it is also found on TRPV1+/ TRPA1+ cutaneous C fibers and in dorsal root ganglia .
Signaling via either the IL-31R or OSMR, a heterodimer composed of the OSMR β protein and a gp130 subunit, can result in cutaneous inflammation as well as keratinocyte proliferation, differentiation, and apoptosis . Mutations in the gene encoding the OSMR β protein underlie familial primary localized cutaneous amyloidosis , an autosomal dominant disorder characterized by chronic localized itching and scratching that results in deposition of keratin-derived amyloid in the dermis . Higher levels of IL-31 expression are found in the skin of patients with atopic dermatitis, prurigo nodularis, and cutaneous T-cell lymphoma (CTCL) . Serum levels of IL-31 have been found to correlate with pruritus severity in patients with advanced CTCL but not in those with atopic dermatitis .
Thymic stromal lymphopoietin (TSLP) is produced by keratinocytes and promotes Th2-type responses. TSLP acts directly on TRPA1-expressing neurons to elicit itch in mice , and TSLP expression is increased within lesions of atopic dermatitis . IL-4 and IL-13 produced by Th2 cells contribute to the itch of atopic dermatitis via activation of JAK (Janus kinase)/STAT (signal transducer and activator of transcription) signaling cascades.
TNF binding to its receptors is known to sensitize nociceptive nerve endings, but its role in itch is unclear. Although targeted TNF inhibitors do not directly decrease pruritus, thalidomide has anti-TNF effects and can be effective in treating the itch associated with prurigo nodularis .
Chronic Itch
Chronic itch may occur in the setting of pruritoceptive itch originating from skin disease, neuropathic itch due to pathology in the nervous system, and systemic or psychiatric disorders (see Ch. 6 ). It often has a significant effect on patients’ quality of life. Chronic itch and chronic pain share several features, with both potentially involving peripheral and central sensitization ( Table 5.4 ; Fig. 5.3 ). Interestingly, familial chronic itch was recently associated with heterozygous variants in the gene encoding the collagen type VI α5 chain, which is expressed in the dermis .
| COMPARISON OF CHARACTERISTICS OF CHRONIC ITCH AND CHRONIC PAIN | ||
|---|---|---|
| Chronic itch | Chronic pain | |
| Peripheral sensitization | Sensitized C nerve fibers | Sensitized C nerve fibers |
| Central sensitization | Alloknesis Hyperknesis | Allodynia Hyperalgesia |
| Neuromediators | Nerve growth factor Neurotrophin 4 | Nerve growth factor Neurotrophin 4 |
| Chemical mediators |
|
|
| CNS areas activated |
|
|
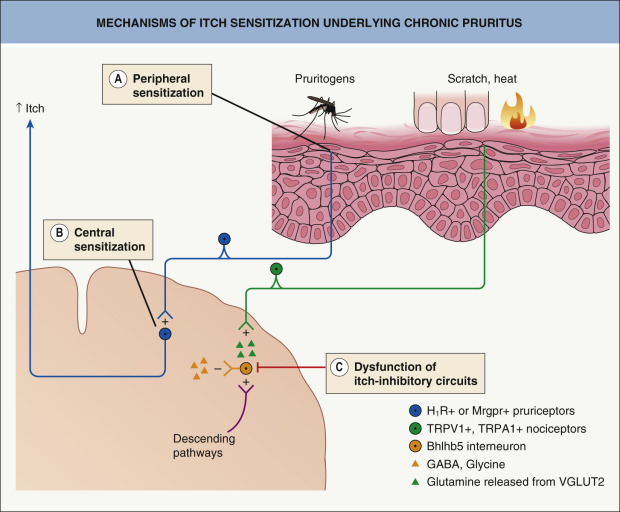
Peripheral Sensitization in Chronic Itch
An increased density of cutaneous nerve endings is observed in some forms of chronic itch. Patients with chronic itch in the setting of atopic dermatitis have increased neurotrophin levels in involved skin, including NGF and neurotrophin 4 . Chronic localized pain is associated with elevated levels of the same neurotrophins, which are known to sensitize nociceptive neurons . In humans, intradermally injected NGF sensitizes non-histaminergic itch , while selective sensitization of non-histaminergic itch was observed in a murine model of chronic dry skin .
Central Sensitization in Chronic Itch
Chronic itch leads to sensitization of second-order neurons within the dorsal horn of the spinal cord (see Fig. 5.1 ). In a mouse model of chronic pruritus, STAT3-dependent reactive astrogliosis within the dorsal horn amplified itching via lipocalin-2 signaling . Spinal TLR4 signaling also plays a role in the spinal astrocyte activation and astrogliosis that underlies chronic itch .
There are two forms of increased sensitivity to itch, alloknesis and hyperknesis. In alloknesis , stimuli that normally do not induce itch such as touch or gentle warming do so in the skin that surrounds a pruritic area . This phenomenon is analogous to allodynia, in which gentle mechanical stimuli give rise to a perception of pain. Like allodynia, alloknesis requires ongoing activity in primary afferent C fibers and is probably mediated by low-threshold myelinated mechanoreceptor Aβ fibers (see Fig. 5.2 ). Alloknesis is common and represents a prominent feature of atopic dermatitis, explaining pruritus associated with dressing and undressing. Hyperknesis is characterized by more intense itch induced by a stimulus that usually produces slight itch and occurs within the skin surrounding an area of inflammation . It is similar to the phenomenon in chronic pain termed hyperalgesia. In mice, neurokinin-1 receptor-expressing spinal neurons play a major role in chronic itch, whereas gastrin-releasing peptide receptor-expressing spinal neurons contribute to hyperknesis, but not alloknesis or ongoing itch .
In patients with chronic itch, painful electrical and heat stimuli may be perceived as itch . An analogous phenomenon can occur in patients with chronic pain, in whom histamine iontophoresis may be perceived as painful . These findings indicate that pain-induced inhibition of pruritus may be compromised in patients with chronic itch. This may also explain why scratching aggravates itch in patients with chronic itch, thereby inducing a vicious itch–scratch cycle.
Itch Related to Impaired Skin Barrier Function
Itch is a common symptom of xerotic skin and is aggravated during the winter in cold climates when the relative humidity falls indoors. Damage to the stratum corneum and the impaired barrier function that results can induce itch even without inflammation. Environmental changes in pH, temperature, and humidity may activate C fibers to transmit the sensation of itch. Cross-talk between the stratum corneum and nerve fibers may explain the pruritus associated with impaired barrier function. Keratinocytes release neuromediators upon damage to the stratum corneum barrier, and nerve fibers sprout in the epidermis in response to this damage. In a murine model of dry skin, enhanced c- fos expression in the CNS was observed, reflecting activation of axons .
Serine proteases that activate PAR-2 and thereby stimulate itch are secreted in response to an increasing (alkaline) stratum corneum pH, which is commonly noted during barrier damage. This suggests that environmental factors that increase stratum corneum pH may increase itch perception . In contrast, a low-pH extracellular environment causes C nociceptors to induce pain.
Senescent Skin and Itch
Itch is particularly frequent in individuals over 65 years of age . Although dry skin is probably the most common trigger, elderly patients can have idiopathic itch without xerosis. Other possible explanations include age-related changes in nerve fibers and central disinhibition of itch due to loss of input from pain fibers. Additional cutaneous changes that may contribute to pruritus as well as xerosis in elderly patients include decreased skin surface lipids and diminished barrier repair.
Cholestatic Pruritus
The enzyme autotaxin and its product lysophosphatidic acid (LPA), a neuronal activator, have a pathogenic role in cholestatic pruritus (see Ch. 6 ). Serum autotaxin levels correlate with itch intensity in patients with cholestasis . In addition, the G protein-coupled bile acid receptor-1 (Gpbar1; TGR5) has been found to have a role in cholestatic pruritus in mouse models.
Treatment of Pruritus
Pruritus can significantly impair quality of life in affected individuals. Acute itch may lead to agitation and difficulty in concentrating, while chronic pruritus can have sequelae such as depression and decreased sexual desire or function .
Pharmacologic Treatments
Unlike pain, for which a host of effective medications are available, there are no general-purpose, consistently beneficial antipruritic drugs. However, recent discoveries of specific neural networks that are involved in itch transmission have led to advances in this relatively unexplored area of medicine . The mechanisms, uses, and side effects of currently available antipruritic drug treatments are outlined in Table 5.5 . Of note, although capable of relieving pruritus by treating inflammatory skin disease, corticosteroids are not intrinsically antipruritic.
| DRUG TREATMENT OF PRURITUS | |||
|---|---|---|---|
| Medication | Mechanism(s) of action | Uses and efficacy | Major adverse effects |
| Topical agents | |||
| Capsaicin (active component in hot chili peppers) |
|
|
|
| Doxepin |
|
|
|
| Menthol |
|
|
|
| Pramoxine |
|
|
|
| Tacrolimus, pimecrolimus |
|
|
|
| “Barrier repair” creams, other emollients and humectants |
|
| None |
| Strontium |
|
| None identified |
| Ketamine-amitriptyline-lidocaine (10%-5%-5% compounded cream) |
|
|
|
| Systemic agents | |||
| Antihistamines (especially doxepin) |
|
|
|
| Naloxone, naltrexone |
|
|
|
| Nalfurafine † |
|
|
|
| Butorphanol |
|
|
|
| Mirtazapine |
|
|
|
| Paroxetine |
|
|
|
| Thalidomide |
|
|
|
| Gabapentin, pregabalin |
|
|
|
| Aprepitant |
|
|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree