Cutaneous Mineralization and Ossification: Introduction
|
Calcium
Calcium is involved in many physiologic processes. It is key to skeletal muscle and myocardial contraction, neurotransmission, and blood coagulation. In addition, it is the primary mineral in the bony skeleton. On the cellular level, its diverse functions include transmission of information into and between cells, regulation of plasma membrane potential, and exocytosis. Only over the last 20 years has its effect on skin been fully appreciated. Calcium regulates major functions in the epidermal keratinocytes including proliferation, differentiation, and cell–cell adhesion.1–6
Regulatory Hormones
At least three regulatory hormones control the ionic calcium concentration in serum: (1) parathyroid hormone (PTH), (2) calcitonin, and (3) 1,25-dihydroxyvitamin D3 (1,25(OH)2D3).
PTH is an 84-amino acid, single-chain polypeptide that is synthesized in the parathyroid glands. Under normal conditions, a decrease in the serum concentration of ionized calcium results in an increase in PTH production, whereas an increase in the serum concentration of ionized calcium results in a decrease in PTH production. In the kidney, PTH increases renal tubular reabsorption of calcium and increases renal clearance of phosphate. PTH also acts directly on the bone to increase the plasma calcium concentration. It does this acutely by mobilizing calcium from bone into the extracellular fluid. Osteocytes and osteoblasts are the presumed target cells for this effect. PTH also stimulates osteoclastic bone resorption, possibly by stimulating osteoblasts to release factors that activate osteoclasts. PTH together with a decreased plasma phosphate concentration stimulates 1α-hydroxylase activity in the kidney, causing an increase in the plasma concentration of 1,25(OH)2D3. 1,25(OH)2D3 increases intestinal absorption of calcium.
Calcitonin is a 32-amino acid polypeptide that is produced by parafollicular or C cells of the thyroid gland. Calcium is the primary stimulant for calcitonin secretion. Calcitonin lowers the serum calcium concentration, primarily through osteoclast inhibition, but whether it plays a major role in serum calcium metabolism outside of the neonatal period in vivo is unclear.
Vitamin D3, or cholecalciferol, is a secosteroid (steroid with a “broken” ring) formed by the opening of the β ring of 7-dehydrocholesterol. In humans, this formation occurs in the basal layer of the epidermis. First, there is an ultraviolet B-mediated conversion of 7-dehydrocholesterol to previtamin D3. Previtamin D3 then undergoes thermal isomerization to form vitamin D3. To become biologically active, vitamin D3 must first be hydroxylated at carbon position 25 in the liver and then at carbon position 1α by the enzyme 1α-hydroxylase in the kidney. 1α-hydroxylase is tightly regulated. PTH and calcitonin increase its activity, whereas calcium, phosphate, and 1,25(OH)2D3 inhibit it.7
1,25(OH)2D3, similar to PTH, increases the concentration of plasma calcium. Its primary action is to stimulate the active transport of calcium across the intestine. 1,25(OH)2D3 also increases the plasma calcium concentration by mobilizing calcium from bone.8 The simultaneous presence of PTH appears to be necessary for this effect.
1,25(OH)2D3 also plays a major role in the growth and differentiation of tissues, including skin.9 1,25(OH)2D3 acts through its nuclear receptor (vitamin D receptor, VDR), which is a member of the superfamily of steroid/thyroid/retinoid nuclear receptors. In the skin, receptors for 1,25(OH)2D3 are present on epidermal keratinocytes, pilosebaceous structures, and in the dermis.10–13 In human keratinocyte cultures, 1,25(OH)2D3 causes a dose-dependent decrease in proliferation, an increase in morphologic differentiation, and an increase in terminal differentiation markers.14 Cultured human keratinocytes can also convert 25(OH)D3 to 1,25(OH)2D3, suggesting that the epidermis may regulate its own growth and differentiation by endogenously produced 1,25(OH)2D3.14 The mechanism by which 1,25(OH)2D3 may induce differentiation of epidermal cells may be through calcium, because calcium is required for terminal differentiation of keratinocytes. 1,25(OH)2D3 may facilitate calcium entry into cells and, through induction of calcium-binding proteins, facilitate the ability of calcium to regulate various cellular processes.
Aberrant Calcification and Ossification
Despite the careful regulation of serum calcium, calcification and ossification of cutaneous and subcutaneous tissues may occur.15 Calcification is the deposition of insoluble calcium salts; when it occurs in cutaneous tissues it is known as calcinosis cutis. Ossification is the formation of true bony tissue by the deposition of calcium and phosphorus in a proteinaceous matrix as hydroxyapatite crystals. Cutaneous calcification may be divided into four major categories: (1) dystrophic, (2) metastatic, (3) idiopathic, and (4) iatrogenic. Dystrophic calcification is the most common type of calcinosis cutis and occurs as a result of local tissue injury. Although calcium and phosphate metabolism and serum levels are normal, local tissue abnormalities, such as alterations in collagen, elastin, or subcutaneous fat may trigger calcification. The internal organs usually remain unaffected. Metastatic calcification is the precipitation of calcium salts in normal tissue secondary to an underlying defect in calcium and/or phosphate metabolism. The calcification may be widespread and, in addition to the skin, affects predominantly blood vessels, kidneys, lungs, and gastric mucosa. All patients presenting with signs of metastatic calcification should receive a calcium and phosphate metabolic evaluation. Idiopathic calcification occurs without identifiable underlying tissue abnormalities, abnormal calcium, and/or phosphate metabolism. Cutaneous calcification also may be iatrogenic. Cutaneous ossification most commonly occurs secondary to local tissue alteration or preexisting calcification. Any calcifying disorder of the skin may ossify secondarily. On rare occasions, primary cutaneous ossification may occur without underlying tissue abnormalities or preexisting calcification.
Dystrophic Calcification
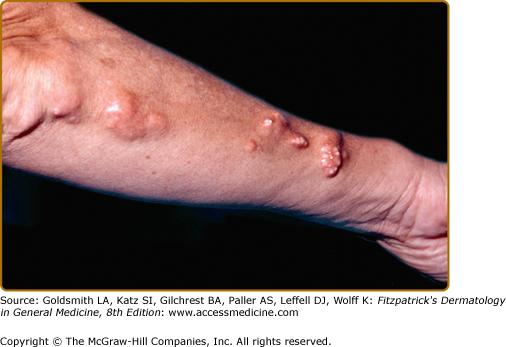
Dystrophic calcification frequently occurs in connective tissue diseases.16 Scleroderma and CREST syndrome (calcinosis cutis, Raynaud phenomenon, esophageal dysfunction, sclerodactyly, telangiectasia) are notable examples that are frequently associated with calcinosis cutis (Fig. 138-1; see Chapter 157). In these disorders, nodules and plaques of calcium deposits may occur in the skin, subcutaneous tissue, muscle, or tendons. The calcium deposits most commonly occur on the upper extremities, especially on the fingers and wrists, but may occur in any area subject to trauma or motion. As the calcifications enlarge, they may ulcerate and exude a chalky material.
Dystrophic calcification also occurs in dermatomyositis (see Chapter 156). It is more commonly associated with juvenile rather than adult-onset dermatomyositis (formerly in about 44% of children as opposed to 20% of adults); nevertheless, the more aggressive early treatment of juvenile dermatomyositis has been associated with a decrease in the occurrence of dystrophic calcification.17 The calcification tends to occur 2–3 years after disease onset and most frequently appears on the elbows, knees, shoulders, and buttocks.18 The calcium deposits may be painful and can ulcerate. They also may exude a chalky material, form sinuses, and become chronically infected. Calcium salt deposition may become quite extensive, progressing along fascial planes of skin and muscle, forming an “exoskeleton,” and leading to significant morbidity and mortality. Calcinosis cutis in dermatomyositis is difficult to treat; however, if the patient survives long enough, the calcified nodules may improve spontaneously. Although uncommon, calcinosis cutis has been described in all clinical subsets of lupus erythematosus.19–22
There is no standard treatment for dystrophic calcification. A diet low in calcium and phosphate along with aluminum hydroxide has been reported to arrest or facilitate regression of the calcified nodules.23 Disodium etidronate has also been used with some success.24 Several series report long-term treatment with diltiazem improves the calcinosis in some patients.25,26 Other reported treatments include warfarin, colchicine, probenecid, and bisphosphonates.16,27 Occasionally, calcium deposits must be removed surgically to clear sinus tracts, ulcers, or chronic infections.
Pancreatic enzyme panniculitis (see Chapter 70) is a lobular panniculitis that commonly demonstrates dystrophic calcification. It occurs in patients with pancreatitis or pancreatic adenocarcinoma and is presumably caused by the action of liberated pancreatic enzymes on subcutaneous fat. The fatty acids formed by lipolysis may combine with calcium and form calcium soap.
In subcutaneous fat necrosis of the newborn, erythematous, well-defined nodules and plaques occur during the first few weeks of life over the cheeks, back, buttocks, and extremities.28,29 The affected infants are generally otherwise healthy, and the nodules and plaques usually clear spontaneously. Occasionally, the lesions calcify, and in a small subset of patients symptomatic hypercalcemia may develop, sometimes several months after birth.
Dystrophic calcification occurs in patients with pseudoxanthoma elasticum (PXE; see Chapter 137). PXE is a hereditary disorder of elastic tissue characterized by progressive calcification of elastin fibers, primarily within the skin, Bruch’s membrane of the retina, and the cardiovascular system. The cause of PXE was recently identified as a mutation in the ABCC6 gene.30 This gene is thought to play a critical role in transmembrane transport. Most patients with PXE have normal calcium phosphate metabolism, but a few have been identified who have abnormal calcium, phosphate, and/or vitamin D metabolism. Patients in this subset may develop metastatic calcification in the form of calcified or ossified tumors, calcification of the falx cerebri, and arterial calcification.31–33
Ehlers–Danlos syndrome (EDS; see Chapter 137) is a group of inherited disorders of fibrillar collagen metabolism. Mutations in the collagen genes or enzymes that regulate collagen biosynthesis have been determined to underlie a number of EDS subtypes.34–36 The skin characteristically shows hyperelasticity and fragility with formation of pseudotumors and large gaping scars. Subcutaneous calcified nodules, termed spheroids may appear, and are thought to represent calcified ischemic fat lobules.37,38 Calcification of healing surgical incisions has also been reported in patients with EDS.39
Dystrophic calcification has been observed in patients with porphyria cutanea tarda (see Chapter 132).40,41 Sclerodermoid plaques with dystrophic calcification have occurred on the preauricular area, scalp, neck, and dorsa of the hands. Ulceration with transepidermal elimination of sheets of calcium is also rarely reported. Other genetic disorders in which calcification may occur include Werner syndrome and Rothmund–Thomson syndrome (see Chapter 139).42,43
Dystrophic calcification occurs in association with a variety of benign and malignant cutaneous neoplasms. Often the neoplasms also show ossification in the surrounding stroma.
Pilomatricomas (see Chapter 119) are the most common cutaneous neoplasms that manifest calcification and ossification. Approximately 75% of pilomatricomas show calcification and 15% to 20% show ossification.44,45 Ossification usually occurs within the connective tissue adjacent to the shadow cells, probably through metaplasia of fibroblasts into osteoblasts. Activating mutations in the adherens junction protein β-catenin have been identified in some pilomatricomas.46
A large number of other neoplasms may be associated with calcification and ossification, including: pilar cyst, basal cell carcinoma, intradermal nevi (probably as a result of inflammation or folliculitis), desmoplastic malignant melanoma, atypical fibroxanthoma, pyogenic granuloma, hemangioma, neurilemmoma, trichoepithelioma, and seborrheic keratoses.47–51 Mixed tumors (chondroid syringomas) may also show calcification and ossification. However, unlike other neoplasms, the ossification occurs within the tumor via ossification of the chondroid cells, much like endochondral bone formation occurring in the epiphyses of bones.
Infectious agents may produce enough cutaneous damage to cause dystrophic calcification. Parasitic infections that may result in calcinosis cutis include onchocerciasis (Onchocerca volvulus) and cysticercosis (Taenia solium).52,53 Calcinosis cutis has also been reported as a complication of intrauterine herpes simplex infection.54
Metastatic Calcification
Metastatic calcification most commonly occurs in chronic renal failure and takes the form of either benign nodular calcification or calciphylaxis. In chronic renal failure, decreased clearance of phosphate results in hyperphosphatemia. In addition, the impaired production of 1,25(OH)2D3 results in a decrease in calcium absorption from the intestine and decreased serum calcium levels. The hypocalcemia results in increased PTH production and secondary hyperparathyroidism. Elevated levels of PTH cause bone resorption and mobilization of calcium and phosphate into the serum, leading to normalization of the serum calcium concentration but marked hyperphosphatemia. If the solubility product of calcium and phosphate is exceeded, metastatic calcification may occur. In benign nodular calcification, the calcifications typically occur at periarticular sites, and their size and number tend to correlate with the degree of hyperphosphatemia. The lesions disappear with normalization of calcium and phosphate levels.
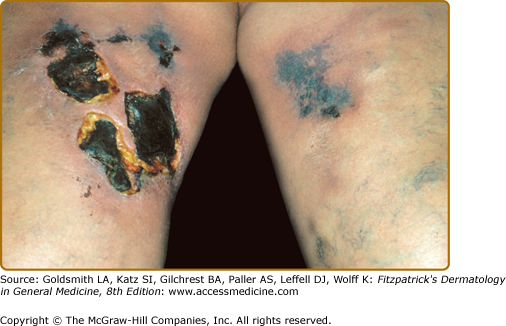
Calciphylaxis is a life-threatening disorder characterized by progressive vascular calcification, soft tissue necrosis, and ischemic necrosis of the skin (see Chapter 151).60 Clinically, it presents as firm, extremely painful, well-demarcated, violaceous plaques associated with soft tissue necrosis and ulceration (Fig. 138-2). The lesions may occur anywhere, but the lower extremities are most frequently involved. The mortality rate is estimated to be approximately 80%. Histopathologically, there is medial calcification of small and medium-sized arteries with intimal hyperplasia, primarily in dermal and subcutaneous tissues. Calciphylaxis occurs almost exclusively in patients with a history of chronic renal failure and prolonged secondary hyperparathyroidism. However, there exist rare reports of the occurrence of calciphylaxis in the absence of renal failure.61 Most of the patients reported are female, and there may be an association with obesity and poor nutritional status.62,63
The pathogenesis of calciphylaxis remains controversial. Experiments in a rat model suggested that calciphylaxis may be triggered by exposure to a sensitizing agent (PTH, dihydrotachysterol, or vitamin D) followed by a challenging agent (metal salts, albumin, or corticosteroids).64 However, the clinical description and histopathology of the animal lesions differ from the human disease. Protein C dysfunction has also been described in a subset of patients with calciphylaxis, but this more likely is a mark for a coagulation defect that predisposes this group to calciphylaxis.65 More recently, it has been proposed that conversion of vascular smooth muscle cells into osteoblast-like cells is a critical step in the development of progressive vascular calcification as is seen in calciphylaxis. This conversion may be stimulated by phosphates, substances that stimulate inflammation in the vascular wall and bone morphogenic protein (BMP)-2. Other proteins that are currently under study as potential effectors, both positive and negative, are BMP-7, osteoprotegerin, matrix Gla protein, fetuin-A, and phosphatonins.66
The current therapy of calciphylaxis involves a multifaceted approach. The calcium-phosphate product should be normalized by methods including: low calcium dialysis, use of phosphate binders that combine calcium acetate and magnesium carbonate, sodium thiosulfate, and parathyroidectomy in those instances where medical management fails.67 Aggressive management of wound infections and judicious use of debridement may help lower the incidence of sepsis and death in these patients.
Chronic ingestion of vitamin D in supraphysiologic doses (50,000–100,000 units/day) may produce hypervitaminosis D.68 The initial signs and symptoms of hypervitaminosis D are attributable to hypercalcemia and hypercalciuria, and include weakness, lethargy, headache, nausea, and polyuria. Metastatic calcification may also occur.
Milk–alkali syndrome is characterized by excessive ingestion of calcium-containing foods or antacids, leading to hypercalcemia.69
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree