Paraneoplastic Dermatoses
|
Cutaneous manifestations of cancer can present with a variety of clinical findings that may reflect direct involvement of malignant cells, an epiphenomenon of a distant malignancy, or in the context of a familial cancer syndrome. The term “paraneoplastic dermatoses” refers to dermatoses (often unique) secondary to a distant cancer. There are two essential criteria: (1) the dermatosis must develop only after the development of the malignant tumor, even though some tumors may be asymptomatic and occult and (2) both the dermatosis and the malignant tumor follow a parallel course in that complete removal of the cancer results in clearing of the dermatosis and recurrence of the cancer causes relapse of the dermatosis. A suggested classification of clinicopathologic categories is shown in Table 153-1.
Paraneoplastic Dermatosis | Major Internal Malignancy | Percent with Cancer | Approach to the Patient | Unique Features |
|---|---|---|---|---|
Hyperkeratotic Diseases | ||||
Acanthosis nigricansa | Adenocarcinomas: intra-abdominal; gastric (50%–60%) | Unknown | CBC, CMP, fasting blood glucose; upper endoscopy or CT scan of abdomen if history supports | Older age, rapid course, and oral involvement more common in malignancy associations. Usually with insulin resistance. |
Acquired ichthyosisa | Hodgkin’s lymphoma (70%–80%) | Unknown | CBC, CXR; consider CT of abdomen to rule out lymphoma | Spares flexures, palms, soles; up to 30% of cases may occur in patients with AIDS |
Pityriasis rotunda | Hepatocellular, gastric carcinoma | 5%–30% | CMP, RPR, HIV | Type I: African/Asian; <30 lesions, high rate malignancy Type II: Caucasians, familial; >30 lesions, cancer rare |
Tripe palms | Lung CA most common; gastric CA second | >90% | CBC, CMP, CT of chest and abdomen | Coexists with acanthosis nigricans in 75% |
Leser-Trelat sign | Adenocarcinomas: GI (47%); lymphoproliferative (20%) | Unknown | CBC, imaging of GI tract | Early onset, eruptive nature, and pruritus are paraneoplastic features; coexists with acanthosis nigricans in 20% |
Bazex syndromea | SCC of upper aerodigestive tract | Nearly 100% | ENT exam; CXR; thoracoabdominal CT | Acral papulosquamous lesions (ears, nose), paronychia, onychodystrophy |
Collagen-vascular Diseases | ||||
Dermatomyositisa | Women: ovarian and breast CA Men: GI and respiratory tract CA | 25%–30% | CBC, CMP, CXR; up to date cancer screenings, bimanual exam in women; CA-125, CT chest, abdomen, pelvis in select cases | Patients older than in idiopathic form; males predominate; refractory or flaring of previously well controlled disease may signal malignancy |
Progressive systemic sclerosis | Lung; SCC of the tongue (25× increased risk) | 3% | Annual oral exam; pulmonary function test | Decreased pulmonary CO diffusion (<70%) risk factor for lung CA |
Reactive Erythemas | ||||
Erythema gyratum repensa | Bronchial CA (32%) | 82% | CXR, upper GI evaluation, mammogram | “Wood-grain”-patterned erythema |
Necrolytic migratory erythema | Pancreatic α-cell tumor | Nearly 100% | Abdominal CT, somatostatin receptor scintigraphy | Not parallel to malignancy |
Neutrophilic Dermatoses | ||||
Sweet’s syndromea | AML and lymphoma (85%) | 20% | CBC with different-bone marrow biopsies if multiple abnormalities seen; skin biopsy for histology and culture | Neutrophilia and fever may not be present in patients with hematologic malignancy; presentation often more severe in this form |
Pyoderma gangrenosum | AML most frequent; multiple myeloma second | 7% | Same as Sweet’s syndrome; SPEP/UPEP | Atypical presentation more common with malignancy; may be on a continuum with Sweet’s syndrome; IgA paraproteinemia 10%–18% |
Dermal Proliferative Diseases | ||||
Multicentric reticulohistiocytosis | No specific cancer type or location | 28% | CBC, CMP, CXR | Not parallel to malignancy |
Necrobiotic xanthogranuloma | IgG paraproteinemia with 10% progressing to multiple myeloma | 80% (gammopathy) | CBC, CMP, SPEP/UPEP | Periorbital involvement especially with necrosis |
Disorders of Dermal Deposition | ||||
Scleromyxedema | Monoclonal gammopathy of undetermined significance; rare multiple myeloma | 80% (gammopathy) | CBC, CMP, ECG, CXR; thyroid studies; SPEP/UPEP | Most have IgG-λ monoclonal gammopathy; only rarely convert to multiple myeloma but poor prognosis if it occurs |
Systemic amyloidosis | Multiple myeloma, thyroid or multiple endocrine neoplasia | 13%–26% (multiple myeloma) | Bx of clinically involved skin, rectal or abdominal fat tissue; SPEP/UPEP | Macroglossia, pinch purpura, carpal tunnel clue to systemic amyloidosis; 1-year mean survival in patients with multiple myeloma |
Bullous Disorders | ||||
Paraneoplastic pemphigus | Lymphoproliferative (75%): non-Hodgkin (42%), CLL (29%) | Nearly 100% | CBC, CMP, CXR, CT abdomen | Parallels benign, but not malignant tumors |
Dermatitis herpetiformis | Gastrointestinal (78%) and non-Hodgkin lymphoma | 4% | Colonoscopy with mucosal biopsy | Occurs significantly more in patients who do not follow a gluten-free diet |
Other Changes | ||||
Hypertrichosis lanuginosa acquisita | Men: lung, colorectal Women: colorectal, lung or breast | Nearly 100% | CXR, colonoscopy, mammogram | Excessive downy hair initially concentrated on face, spreading caudally |
Trousseau’s syndrome (migratory thrombophlebitis) | Lung and pancreatic carcinoma | 50% | CBC, CXR, CT of abdomen | Responds best to low molecular weight heparin |
aDermatoses established to closely parallel course of malignancy. | ||||
Changes of the skin can be markers of an internal malignancy, but the exact prevalence is unknown. This is in part due to the complexity of disease variation in some cases and the rarity of occurrence in others. Age, sex, and risk factors for developing a paraneoplastic dermatosis correlate with the overall risk for the respective internal malignancy.
The specific relationship of the cancer with the marker can be variable. For instance, hypertrichosis lanuginosa acquisita can be a marker for many different malignant neoplasms, whereas necrolytic migratory erythema (NME) is nearly specific for a glucagon-producing tumor of the pancreas. The specific correlations with internal malignancies for each paraneoplastic dermatosis are listed in Table 153-1.
Paraneoplastic syndromes are often grouped by their major clinical presentations, and several classification systems exist. Unfortunately, our lack of knowledge prevents a more pathophysiologic approach.
Proven association of the cutaneous eruption with a tumor can be more easily made when the supposed manifestation is very rare and the tumor is also very uncommon. It becomes a major problem, however, when the manifestation is very common, such as the seborrheic keratoses in the sign of Leser-Trélat, and the presumed association is with a wide spectrum of commonly occurring neoplasms. In this situation, anecdotal reports abound.
Hyperkeratotic Dermatoses
(See also Chapter 151)
|
The term acanthosis nigricans was originally proposed by Unna, although the first cases were malignancy associated and described independently by Pollitzer and by Janovsky in 1890. Curth clinically classified acanthosis nigricans into malignant, benign, syndromic acanthosis nigricans or pseudoacanthosis nigricans (obesity related). Today acanthosis nigricans is classified into two broad categories: (1) benign (familial, obesity related, hyperinsulinemic states, autoimmune disease associated) and (2) malignant. Malignant acanthosis nigricans typically occurs in older patients and frequently coexists with other paraneoplastic dermatoses such as tripe palms and the sign of Leser-Trélat.
The majority (80%) of acanthosis nigricans occurs idiopathically or in benign conditions such as endocrinopathies and/or heritable diseases.1 Malignancy-associated acanthosis nigricans is rare. It was present in 1 of 35 patients with intrathoracic or intraabdominal malignancies,2 and in 2 of 12,000 cancer patients. Although reported in all age groups, at least 80% of malignancy related acanthosis nigricans occurs in individuals over the age of 40.1
Although the precise etiology of benign acanthosis nigricans remains unclear, there is evidence that insulin plays a significant role (see Chapter 151). The pathogenesis of malignancy-associated acanthosis nigricans is even less clear. A humoral factor produced by the tumor is likely, given cases in which the skin disorder improved or resolved following treatment of the malignancy. Elevated urinary transforming growth factor (TGF)-α and increased expression of epidermal growth factor (EGF) receptors in lesional skin were noted in a patient with acanthosis nigricans, acrochordons, Leser-Trélat sign, and melanoma. The enhanced expression of this cytokine and its receptor normalized after removal of the melanoma, and the acanthosis nigricans, acrochordons, and seborrheic keratoses improved postoperatively.3 A separate case, with gastric cancer, was also shown to express TGF-α and EGF receptors.4
More recently, a pathogenic role has been proposed for a tyrosine kinase receptor expressed on the basal cells of the epidermis. In several inherited human skeletal dysplasias associated with acanthosis nigricans, such as craniosynostoses and dwarfing chondrodysplasias, activating mutations in the fibroblast growth factor receptor 3 (FGFR3) can be observed, whereby FGFR3 has an inhibitory affect on bone formation.5 In other studies, FGFR3 has oncogenic affects in such malignancies as multiple myeloma, bladder, and cervical carcinoma. With respect to the role of FGFR3 on the epidermis, animal models with activating mutations develop benign epidermal tumors resembling acanthosis nigricans and seborrheic keratoses.6
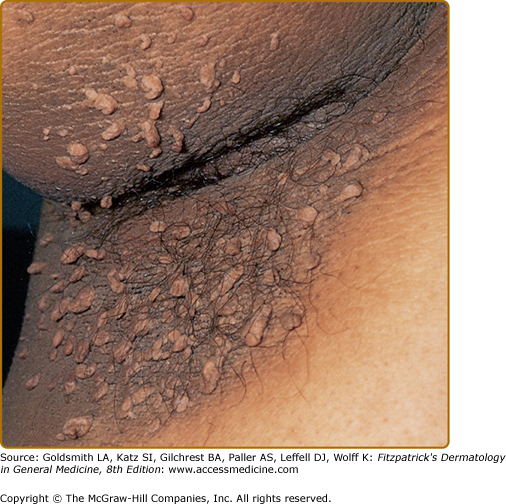
(See also Chapter 151). The clinical hallmark of acanthosis nigricans is development of gray-brown, velvety plaques that may start as a dirty appearance. The hyperpigmentation is later accompanied by hypertrophy, increased skin markings, and papillomatosis. Pruritus can be a problem in some patients. The most commonly involved locations are the axillae, neck, external genitalia, groin, face, inner thighs, antecubital and popliteal fossae, umbilicus, and perianal area. Acrochordons may develop, superimposed on the acanthosis nigricans or on other locations (Fig. 153-1).
Several inherited syndromes have acanthosis nigricans described as a feature of the disease. Those that have been reported are listed in Box 153-1.
|
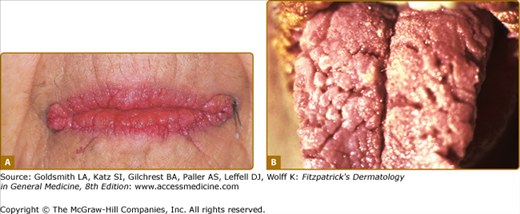
The lesions of malignant and benign acanthosis nigricans are indistinguishable. However, there are subtle differences regarding the age of onset, distribution, and speed of onset that may assist in recognizing the paraneoplastic form. The benign form typically presents at a younger age and has a gradual progression of flexural surfaces. Concerns for malignancy-associated acanthosis nigricans should arise when a rapid appearance of the lesions in an older individual along with atypical sites such as the oral mucosa is involved (Fig. 153-2). For the clinical differential diagnosis of acanthosis nigricans, see eBox 153-1.1.
Figure 153-2
Acanthosis nigricans. A. Verrucous and papillomatous growths of the vermillion border of the lip. B. Velvety thickening of the tongue. (A and B are not the same patient.) (A and B: From Wolff K, Johnson RA: Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology, 6th edition. New York, McGraw-Hill, 2009)
Most Likely
Consider
Always Rule Out
|
Many consider tripe palms (see below) and the sign of Leser-Trélat (see below) to be along a spectrum of malignant acanthosis nigricans given the high degree of co-occurrence and histologic similarities. Florid cutaneous papillomatosis is also a part of this same spectrum. It presents as a rapid appearance of verrucous papillomas that are identical in appearance to typical viral warts. This phenomenon occurs with malignant acanthosis nigricans and in a large series had a 100% correlation with an internal malignancy, most commonly gastric adenocarcinoma.7
Many types of malignancies have been reported in association with malignant acanthosis nigricans. A review of the literature has shown up to 90% of patients have an associated intra-abdominal adenocarcinoma, of which approximately 60% are gastric.8
Histopathology of acanthosis nigricans classically shows hyperkeratosis and epidermal papillomatosis. There is only slight acanthosis and in some places, atrophy can be seen. Other features that can be observed include pseudocyst formation and increased melanin pigmentation. The dermis is usually devoid of inflammatory cells with the exception of mucosal acanthosis nigricans, which may have a mixed infiltrate of lymphocytes, plasma cells, and occasional neutrophils. The pathologic differential diagnosis includes confluent and reticulated papillomatosis of Gougerot and Carteaud, seborrheic keratosis, and epidermal nevus.
The cutaneous complications of acanthosis nigricans are most often of cosmetic concern. With time, severe papillomatosis and acrochordon formation can lead to frictional irritation and can be quite problematic. Of most concern with these patients, however, is an undiagnosed endocrinopathy or malignancy.
The malignant form may also parallel the course of the underlying malignancy. Unfortunately, most associated malignancies present at an advanced stage, and thus the median survival rate for those with malignant acanthosis nigricans has been reported to be from 10–24 months.9
Management of any co-occurring disease or malignancy often improves and may even resolve acanthosis nigricans. Topical keratolytics, including the retinoids, and oral retinoids can reduce the appearance of acanthosis nigricans. Other oral medications reported to show improvement include dietary fish oil, metformin, and cyproheptadine, possibly by inhibition of tumor secreted growth factors in the case of the latter.10
Unfortunately for those with acanthosis nigricans associated with inherited conditions and malignancy, there is no effective prevention.
(See also Chapter 49)
The sudden onset of ichthyosis in an adult may indicate an occult malignant tumor, most often Hodgkin’s lymphoma. However, patients with AIDS can represent up to 30% of patients with acquired ichthyosis.11 The ichthyosiform variant of mycosis fungoides should also be considered as it can rarely be the sole manifestation.12 Diffuse rhomboidal scaling is observed on extensor surfaces and typically spares skin folds, palms, and soles. Erythema is often present in the ichthyotic fissures. Although the ichthyosis usually occurs as a late manifestation of a lymphoma, it may precede the diagnosis by several years and typically parallels the course of the malignancy.
Pityriasis rotunda may be considered a variant that presents as strikingly discrete circular patches with ichthyosiform scale. Individual lesions are usually less than 3 cm but may become confluent and most often involve the trunk and proximal extremities. Paraneoplastic pityriasis rotunda is most commonly associated with hepatocellular and gastric carcinoma. There also exists a familial variant that is not reported to be associated with malignancy.13,14 The pathogenesis is felt to be related to malabsorption resulting in a hypovitaminosis A and a possible contribution by tumor-secreted EGFs. Treatment is with emollients, retinoids, and α-hydroxy acids.
Tripe palms are a rare paraneoplastic dermatosis with approximately 100 cases reported in the literature. The association with malignancy is high with a greater than 90% occurrence.
Specific mechanisms of development have not been fully elucidated. Similar to acanthosis nigricans, there is limited evidence for the role of TGF-α inducing cellular proliferation in the pathogenesis of tripe palms, and that normalization of this cytokine results in clinical improvement.15
Other investigations have shown melanocortin-1 and EGFs to be upregulated in malignancy-associated tripe palm.16,17 However, these are not uniformly present.18
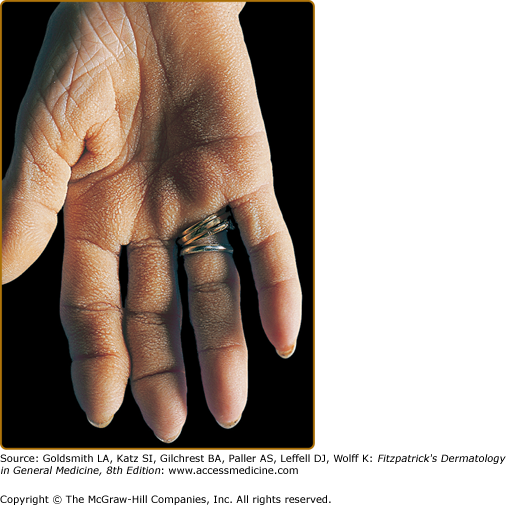
The name originated from the description in the first reported case in 1963 whereby the patient suggested his hands looked similar to the rugose surface of the bovine stomach (Fig. 153-3).19 Based on clinical similarities and frequency of co-occurrence, many consider this to be a form of palmar acanthosis nigricans. Indeed, tripe palms have also been called “acanthosis nigricans of the palms” and “acanthosis palmaris.” The honeycombed and corrugated thickening of the palms may be associated with periungual tenderness. Normal dermatoglyphic ridges are accentuated. As a clinical differential diagnosis, the palmar morphology can be similar in keratitis–ichthyosis–deafness (KID) syndrome (see Chapter 49).
Histopathology of tripe palms includes hyperkeratosis, acanthosis, and papillomatosis. These features closely resemble those pathologic findings observed in acanthosis nigricans and seborrheic keratoses. Additional findings can include dermal mucin and mast cells in approximately 20% of specimens.
The majority (75%) of patients will have concomitant acanthosis nigricans. In patients with only tripe palms, pulmonary carcinoma (53%) is the most frequent malignancy, especially squamous cell type. In patients with both tripe palms and acanthosis nigricans, gastric carcinoma (35%) is the most common followed by lung carcinoma (11%).20
This is often a complication itself of underlying malignancy.
A complete malignancy work up should be performed in patients with tripe palms due to the high association and because the appearance precedes the diagnosis of malignancy in approximately 40% of cases.20
There is no specific therapy for tripe palms. Similar to acanthosis nigricans, there are anecdotal reports of improvement with oral retinoids alone and in combination with metformin.21 Approximately 30% will resolve with treatment of the underlying tumor.22 However, there are many cases whereby a remission of the cancer has had no effect.
There is no way to prevent the development of tripe palms. Patients should be up-to-date with standard cancer screenings. An expanded cancer investigation is warranted if no coexistence of malignancy is noted at the time of diagnosis.
|
The sign of Leser-Trélat is a rare paraneoplastic dermatosis that occurs with equal frequency between men and women and among different races. Similar to the occurrence of malignancy, this sign is more common in older individuals.
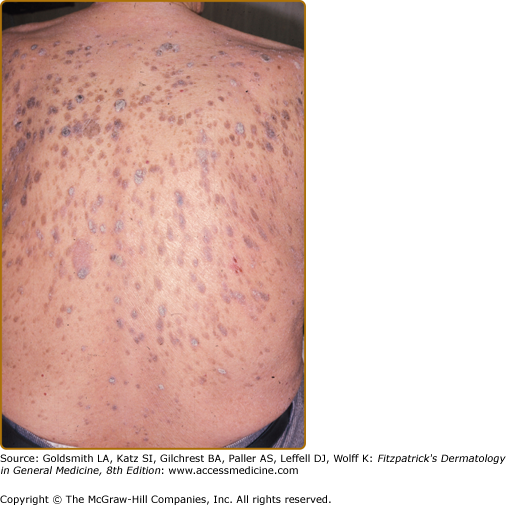
Controversy has surrounded this as a true diagnosis due to the extremely common nature of benign seborrheic keratoses and furthermore, occurring in the age group most susceptible to malignancy. In a large population based study of 1,752 consecutive cases of seborrheic keratoses, there was no statistical evidence of an increased incidence of internal malignancy compared to the general population. Subanalysis of those presenting with eruptive seborrheic keratoses also failed to demonstrate an increased risk of internal malignancy.23 In other large studies of patients with seborrheic keratoses and a recent solid tumor, a comparison with age- and sex-matched controls have not demonstrated a difference in either the clinical features or numbers of seborrheic keratoses.24,25 As such, large epidemiologic studies have not provided the evidence needed to conclusively define this sign as a true paraneoplastic dermatosis.
Although large studies have not shown a statistical difference, there exists evidence to demonstrate that this sign may signify an internal malignancy. First, there are cases of eruptive seborrheic keratoses in patients in their 20s, an age group that rarely exhibits these lesions. Further workup in both cases led to a diagnosis of an internal malignancy.26,27 Clinically, Leser-Trélat sign often coexists with malignant acanthosis nigricans, a more established paraneoplastic phenomenon. Additionally, alterations in growth factor expression differ from control patients. Together, this provides evidence that the Leser-Trélat sign is a legitimate but extremely uncommon paraneoplastic dermatosis.
An exact pathogenesis has yet to be elucidated. However, similar to acanthosis nigricans and tripe palms, evidence also exists with the Leser-Trélat sign that an alteration in growth factor homeostasis is contributory. In several instances, a state of increased growth factor expression has been observed with increased urinary levels of the growth factors EGF and TGF-α detected in patients with an underlying malignancy and eruptive seborrheic keratoses. Subsequently, growth factor levels decreased following primary tumor resection.28
It is not known if the tumors are the primary source or are inducing a secondary secretion of such growth factors as TGF-α and EGF. In an analysis of a patient with pancreatic carcinoma and the Leser-Trélat sign, the tumor cells expressed low levels of EGF while the hyperkeratotic lesions of seborrheic keratoses expressed increased EGF.29 In a comparison of Leser-Trélat lesional skin and that of control seborrheic keratoses, increased levels of the growth factor homologs EGF and HER-2/neu were detected in the downward branching epithelial strands.30 In addition to increased levels of growth factor expression, lesional skin in paraneoplastic acanthosis nigricans and seborrheic keratoses has alterations in the extracellular matrix.31 It is unknown what the direct impact of growth factor signaling has on the skin. Proposed ideas include either inducing a hyperproliferative state primarily or by altering the surrounding environment such as via the extracellular matrix. These similar mechanisms further support the idea of a continuum between acanthosis nigricans and the Leser-Trélat sign.
Clinical features signifying a paraneoplastic phenomenon include patients with widespread eruptive seborrheic keratoses. Pruritus is a significant feature occurring in 43%.1 Co-occurrence with other hyperkeratotic paraneoplastic dermatoses is common with 20% having acanthosis nigricans. There are several reports of Leser-Trélat sign and tripe palms coexisting in the same patient.8,32–34 The rapid occurrence of pruritic eruptive seborrheic keratoses, especially in a setting of acanthosis nigricans should alert the clinician to a potential internal malignancy (Fig. 153-4). For the differential diagnosis, see Box 153-2.
Most Likely
Consider
Always Rule Out
|
Adenocarcinomas account for the majority of malignancies described with the sign of Leser-Trélat. Of 130 malignancies reported with this sign, 47.7% involve the gastrointestinal tract.29 The second most common malignancy is lymphoproliferative disorders, and includes leukemias, lymphomas, Sézary syndrome and mycosis fungoides. Involvement of other organs such as the pancreas, kidneys, ovaries, and lungs has been reported in several cases as well. In a series of 44 patients with lung cancer, the sign of Leser-Trélat was observed in 2.27%.35
Typically defined by a concurrent malignancy, the sign of Leser-Trélat has also been reported in non-neoplastic tumors, HIV, conditions of erythroderma and in association with the chemotherapeutic drug cytaribine.36–39 The term “pseudosign of Leser-Trélat” has been used to designate nonmalignancy associated eruptive seborrheic keratoses that presents identically to its paraneoplastic counterpart. Unfortunately, these examples do not help to further define an already confusing and controversial clinical entity.
As with many paraneoplastic dermatoses, sudden reappearance may herald tumor recurrence, although this particular sign only occasionally parallels the malignancy.
This sign can develop from 5 months prior to 9.8 months after the diagnosis of malignancy and once diagnosed, the prognosis is poor with an average survival time of 10.6 months.40 This eruption parallels the course of the underlying malignancy in some cases, but not as a general rule.
Treatment should be directed toward the underlying tumor. If the lesions are symptomatic or cosmetically concerning to the patient, other options for treatment include standard therapies for seborrheic keratoses such as α-hydroxy acids, retinoids, trichloracetic acid, cryosurgery with or without curettage, dermabrasion, laser, and shave excision.
There is no effective prevention other than keeping up with age-appropriate cancer screenings.
|
Bazex established an association between this uniquely distributed papulosquamous eruption and underlying malignancy.41 There have been over 100 cases reported in the medical literature. Patients are typically men over the age of 40. Acrokeratosis paraneoplastica is unrelated to Bazex-Dupre-Christol which is a genodermatosis characterized by congenital hypotrichosis, follicular atrophoderma, and basal cell carcinomas arising at an early age.
The mechanism by which this characteristic eruption occurs is still speculative. One theory proposes that antibodies mounted against the tumor cross react with keratinocyte or basement membrane antigens (similar to those found in paraneoplastic bullous pemphigoid).41 Other theories include a T-cell-mediated immune response to tumor-like antigens in the epidermis or by excessive expression of autocrine growth factors from the tumor resulting in epidermal hyperplasia.42–44 Other hypotheses include low serum levels of vitamin A and zinc.
The name, acrokeratosis paraneoplastica, is aptly descriptive because characteristic lesions are located acrally and exhibit hyperkeratosis. The plaques are usually erythematous to violaceous with overlying scale. Almost all patients have lesions affecting the ears and nails during the disease. Nearly two-thirds will demonstrate involvement of the nose and fingers, and over half have lesions on the hands and feet, especially volar surfaces.45 Other clinical findings include hyperpigmentation and bullae. Bullae, when present, are most common on the hands and feet.
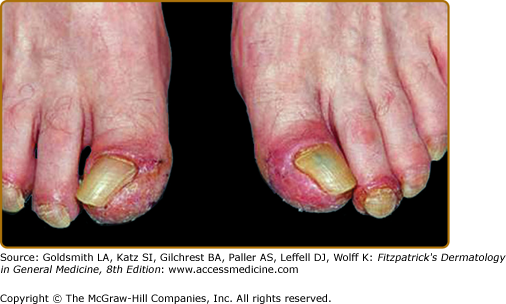
Three stages of skin lesions parallel the growth and dissemination of the underlying tumor. In the first stage, the helices, nose, fingers, and toes are usually affected in a symmetrical fashion. Early lesions are macular and not clearly demarcated, simulating a nonspecific dermatitis. They may exhibit crust in addition to scale. The eruption is classically asymptomatic, but pruritus may be a problem. Paronychia is the first sign of nail involvement (Fig. 153-5). In most cases, the responsible tumor is occult at this stage and remains so for an average of 11 months.45
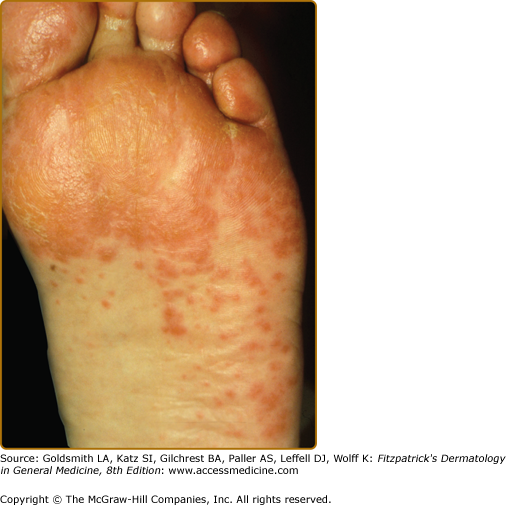
During the second stage the tumor exhibits symptoms, due to local extension or metastatic spread, and the skin eruption becomes more extensive. The cheeks display the typical red to purple scaly or crusted plaques. The palms and soles develop a keratoderma that often spares the central volar surfaces but may lead to painful fissures (Fig. 153-6). Nail plate changes include yellowing, thickening, onycholysis and ridging, both horizontal and vertical.
The final stage is observed when the tumor goes untreated or fails to respond to treatment. All of the above signs and symptoms persist while the papulosquamous lesions begin to appear on the trunk, elbows, knees, and dorsal hands and feet. Rarely, vesicles and bullae may be present, most commonly on the fingers, hands, and feet. Nail changes can be quite variable, ranging from the typical thickening to nail atrophy and loss.
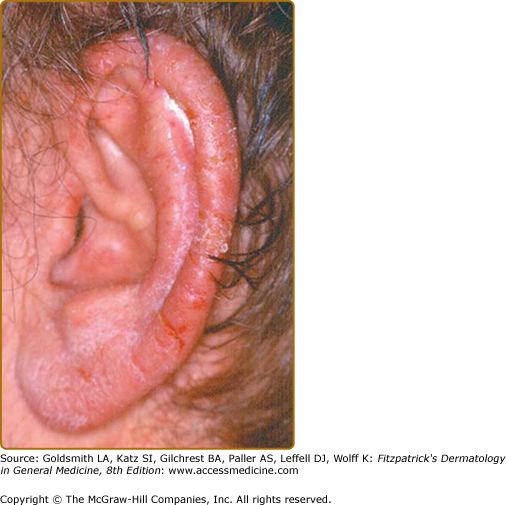
The main differentiation to be made in diagnosis is psoriasis, especially acral variants. The distinguishing clinical feature, which is nearly always present in acrokeratosis paraneoplastica, is involvement of the helices of the ears (Fig. 153-7) and the tip of the nose. Knees and elbows may develop plaques but it occurs late in the disease. Typically, Bazex is treatment resistant unlike other diseases considered in the differential (see Box 153-3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree