Cutaneous Manifestations of Human Immunodeficiency Virus Disease: Introduction
|
The introduction of antiretroviral therapy (ART) has markedly altered the life expectancy and quality of life for many of the 33.4 million individuals worldwide infected with human immunodeficiency virus (HIV).1 However, the number of newly diagnosed infections remains high, and many individuals in areas of high HIV prevalence remain unaware of their infection. Globally, 2.7 million new infections were estimated to have developed in 2008, while 56,300 new infections were estimated to have occurred in United States in 2006.1, 2 It is estimated that 21% of the 1.1 million infected HIV individuals in the United States are currently undiagnosed.2,3 This suggests that identification of HIV infection/acquired immune-deficiency syndrome (AIDS)-associated dermatoses has the potential to facilitate the diagnosis of HIV infection not only in resource-limited settings but in relatively resource-abundant ones.
Cutaneous disorders occur in nearly every patient during the course of HIV disease, either as a result of acquired immunodeficiency or from treatment. The spectrum of dermatologic manifestations of HIV disease is broad and diverse.4 Individuals who have access to combination ART have a markedly altered course of disease if immune restoration is successfully achieved.5 In most cases, there is a marked reduction in the incidence of opportunistic infections and neoplasms. Globally, however, the majority of HIV-infected individuals lack access to ART and, consequently, many of the cutaneous manifestations associated with HIV disease become chronic and progressive.
Etiology
HIV is a lymphotropic human retrovirus, which is predominantly transmitted through sexual contact. Other important means of transmission include exposure to infected blood (including needles shared by injecting drug users and “skin popping”) and transmission from an infected mother to her infant during pregnancy, delivery, or breastfeeding. HIV-1 is the most common cause of HIV infection globally, whereas HIV-2 infection has been detected mainly in West Africa. Although both HIV subtypes cause clinically similar disease, HIV-2 is associated with slower progression of immunosuppression, decreased infectivity, and resistance to non-nucleoside reverse transcriptase inhibitors.6–8
Pathogenesis
The profound immunosuppression that defines HIV disease results from progressive depletion of CD4+ T lymphocytes. Efficient infection of a target cell by HIV requires not only expression of a CD4 molecule on that cell’s surface, but also the presence of a co-receptor (such as CCR5 or CXCR4). Although HIV infects primarily CD4+ T lymphocytes and CD4+ cells of monocytic lineage, any cell that expresses CD4 and an appropriate co-receptor may be infected by HIV.9 The depletion of CD4+ T cells in HIV disease is believed to be multifactorial, and includes direct infection and destruction of CD4+ T cells by HIV, as well immune exhaustion and apoptosis of T cells due to persistent and aberrant activation of the immune system.9
Natural History
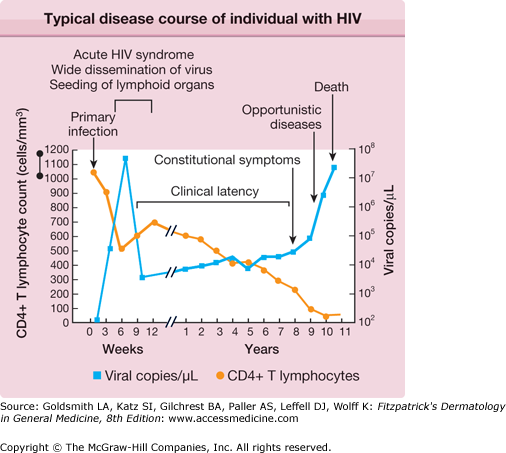
HIV disease is a continuum that progresses from primary infection to death via a sequence of opportunistic infections and neoplasms that mark the gradual deterioration of the immune system (Table 198-1 and Fig. 198-1). Increased availability of ART has dramatically altered the course of HIV disease in many individuals—by delaying the development of symptomatic disease, reducing the burden of opportunistic disease, and prolonging life expectancy. The field of HIV medicine continues to rapidly evolve, and excellent Web resources that are frequently updated include and http://www.cdcnpin.org.
Stage and Clinical Features | Typical Duration | CD4+ Cell Range (Cells/μL) |
|---|---|---|
Acute retroviral syndrome (brief mononucleosis-like illness) | 1–2 weeks | 1000–500 |
Asymptomatic (no symptoms or signs other than lymphadenopathy) | >10 years | 750–500 |
Early symptomatic (non–life-threatening infections, chronic or intermittent illness) | 0–5 years | 500–100 |
Late symptomatic (acquired immunodeficiency syndrome; increasingly severe symptoms, life-threatening infections and cancers) | 0–3 years | 200–50 |
Advanced (increasing risk of death, fewer transferable opportunistic infections) | 1–2 year | 50–0 |
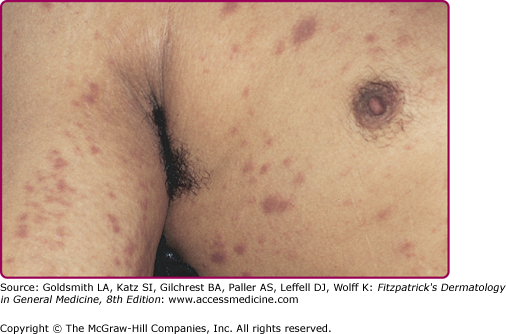
About 3–6 weeks following primary infection, the majority of individuals infected with HIV develop an acute mononucleosis-like syndrome, which is referred to as acute retroviral syndrome. Clinical manifestations include fever, lethargy, rash (Fig. 198-2), myalgias/arthralgias, cervical and axillary lymphadenopathy, pharyngitis, night sweats, and nausea/vomiting/diarrhea. Less commonly reported findings include leukopenia, thrombocytopenia, weight loss, aseptic meningitis, anorexia, abnormal liver function tests, and oral and genital ulcers.10 Symptoms generally last an average of 2–3 weeks and resolve gradually as levels of plasma viremia decrease. It is important to maintain a high index of suspicion in individuals with a suggestive constellation of symptoms. Studies have documented that up to 25% of those with HIV infection are not tested, despite suggestive symptoms.11 In fact, 2% of individuals thought to have Epstein–Barr virus were found to have HIV when tested retrospectively in one study, and roughly half of those had acute primary HIV infection.12
The laboratory diagnosis of HIV-1 infection is typically made by either identification of antibodies to HIV or direct detection of HIV antigens or nucleic acids (Table 198-2). A delay of 3–4 weeks typically occurs between newly acquired HIV-1 infection and development of antibodies, which is referred to as the “window period.” However, core structural protein p24 antigen may be detected several weeks prior to seroconversion. The viral-RNA assay detects infection up to 5 days earlier than the p24 assay, and appears to be more sensitive.11
Test | Component Tested | Window Period | Role in Diagnosis |
|---|---|---|---|
Enzyme-linked immunosorbent assaya | Antibodies (IgM and IgG) | 3–6 weeks | Screening |
Antigen captureb | HIV p24 antigen | 2–3 weeks | Screening |
Western blotting | Antibody (IgG) | 3 weeks | Confirmatory |
Immunofluorescence | Antibody (IgG) | 3 weeks | Confirmatory |
Nucleic acid testing | HIV RNA or DNA | 2 weeks | Confirmatory |
Viral culture | Virus, usually from peripheral blood mononuclear cells, not serum or plasma | — | Confirmatory, research |
The length of time between initial infection and the development of symptomatic disease varies significantly, and is now augmented by ART in many cases. This period of clinical latency does not necessarily imply disease latency. Viral replication may persist, and CD4+ T cell levels progressively decline (Fig. 198-1). The more severe and life-threatening complications of HIV disease typically occur when CD4+ T cell counts fall below 200/μL. The World Health Organization published in 2006 criteria for clinical staging in individuals with confirmed HIV infection, while the US Centers for Disease Control and Prevention (CDC) published a revised classification system for HIV infection in 1993.13,14 The AIDS is defined by the CDC as an HIV-seropositive individual with a CD4+ T cell count <200/μL, a CD4+ T cell percentage <14, or any of several diseases considered to be indicative of a severe defect in cell-mediated immunity.14 Conditions deemed by the CDC to be AIDS-defining in a patient with a confirmed HIV infection are listed in Box 198-1.
Candidiasis of bronchi, trachea, or lungs Candidiasis, esophageal Cervical cancer, invasive Coccidioidomycosis, disseminated or extrapulmonary Cryptococcosis, extrapulmonary Cryptosporidiosis, chronic intestinal (>1 month’s duration) Cytomegalovirus, extrapulmonary Cytomegalovirus disease (other than liver, spleen, or nodes) Cytomegalovirus retinitis (with loss of vision) Encephalopathy, HIV-related Herpes simplex: chronic ulcers(s) (>1 month’s duration); or bronchitis, pneumonia, or esophagitis Histoplasmosis, disseminated or extrapulmonary Isosporiasis, chronic intestinal (>1 month’s duration) Kaposi sarcoma Lymphoma, Burkitt or immunoblastic (or equivalent term) Lymphoma, primary, of brain Mycobacterium tuberculosis, any site (pulmonary or extrapulmonary) Mycobacterium, other species or unidentified species, disseminated or extrapulmonary Pneumocystis jiroveci pneumonia Pneumonia, recurrent Progressive multifocal leukoencephalopathy Salmonella septicemia, recurrent Toxoplasmosis, brain Wasting syndrome due to HIV |
Clinical Findings in HIV Disease
CD4 T Cell Count >500/μL | 500/μL >CD4 T Cell Count >250 μL | CD4 T Cell Count <200/μL |
|---|---|---|
Acute retroviral syndrome | Dermatophyte infections, recurrent or persistent | Bacillary angiomatosis |
Herpes zoster infection (nondisseminated) | Oral candidiasis | Hyperkeratotic scabies |
Seborrheic dermatitis | Oral hairy leukoplakia Herpes zoster infection, disseminated | Cutaneous miliary tuberculosis Eosinophilic folliculitis |
Herpes simplex virus infection (>1 month’s duration) | ||
Idiopathic pruritus | ||
Invasive fungal infections | ||
Kaposi’s sarcoma | ||
Molluscum contagiosum, large facial lesions | ||
Papular pruritic eruption of HIV |
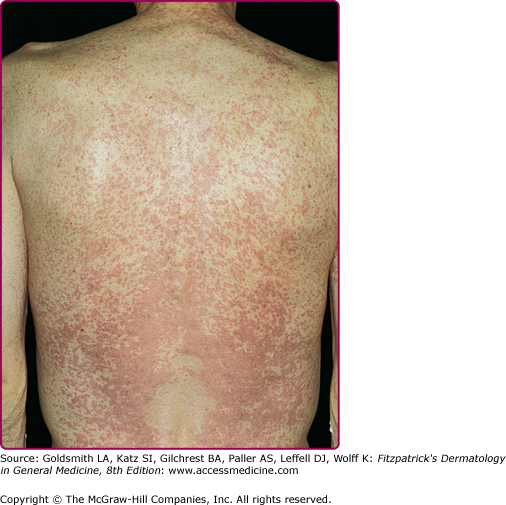
The majority of individuals with acute retroviral syndrome develop a mucocutaneous eruption, typically consisting of distinct, well-demarcated, nonpruritic macules and papules favoring the upper chest and back (particularly the clavicular region), forehead, and scalp (Fig. 198-2).23 Diffuse urticaria and pustular/vesicular eruptions have also been reported, though less commonly. Noninfectious ulcerations of the oral, genital, and anal mucosa are another common finding, and have been reported in up to 6%–28% of patients. Cutaneous symptoms are often accompanied by fever, lethargy, lymphadenopathy, and joint pain. The eruption of acute retroviral syndrome typically presents 3–6 weeks after initial infection, and persists for about 5–8 days. The differential diagnosis of acute retroviral syndrome includes Epstein–Barr virus infection, cytomegalovirus infection, primary herpes simplex infection, viral hepatitis, secondary syphilis, and systemic drug hypersensitivity. As acute infection with HIV-1 has been associated with a high degree of viremia within 2–6 weeks of infection, identification of individuals with acute retroviral syndrome provides the opportunity to prevent secondary transmission during a period of significant viremia prior to seroconversion.
Initiation of ART typically results in partial recovery of the immune system, as measured by an increase in CD4+ T cell counts and decrease in plasma HIV viral load. In general, this leads to clinical improvement; however, in a small subset of individuals, a paradoxical clinical deterioration occurs. Termed immune restoration disease or immune reconstitution inflammatory syndrome (IRIS), this phenomenon typically presents within the first 3 months of ART initiation with either symptoms of a new infectious or inflammatory condition or clinical worsening of an existing infectious or inflammatory condition. It is believed that IRIS results from an exaggerated immune response to preexisting microbial, host, or other antigens at a time when CD4+ T cell counts are rapidly increasing. IRIS has been reported in 15%–25% of individuals initiating ART, with an incidence of 15%–45% in individuals who also have a documented underlying opportunistic infection.24 Risk factors associated with the development of IRIS include male gender, a shorter interval between initiating treatment of an opportunistic infection and starting ART, a rapid fall in HIV viral load after initiation of ART, a lower baseline CD4+ T cell count, and a higher baseline viral load during ART.25,26 IRIS has been reported in association with a broad range of infections, inflammatory disorders, and neoplasms (Box 198-2).27,29 If a patient presents with paradoxical worsening of an existing disease in the setting of starting ART, it is important to consider the possibility of IRIS, in addition to treatment nonadherence, antimicrobial resistance, and tachyphylaxis. Although most cases of IRIS are self-limited and typically last weeks to months, IRIS can be associated with severe morbidity and, in rare cases, even mortality.
Infectious Mycobacterial infection Cytomegalovirus Viral hepatitis Varicella zoster virus Herpes simplex virus Human papillomavirus Cryptococcosis Histoplasmosis Leishmaniasis Inflammatory Sarcoidosis Rheumatoid arthritis Systemic lupus erythematosus Tumid lupus Dyshidrotic eczema Eosinophilic folliculitis Neoplastic Kaposi sarcoma Dermatofibroma |
Management of IRIS consists of continuation of antiretroviral medications and initiation of appropriate therapy for the underlying infectious or inflammatory condition. In addition, systemic steroids have been reported to be beneficial in some cases.33 In severe cases of IRIS, discontinuation of ART should be considered and weighed against the risk of development of viral resistance or HIV progression.
The introduction of ART has markedly altered the natural history of HIV infection by allowing for immune reconstitution, thereby significantly reducing the incidence of both opportunistic infections and neoplasms. As a result, many of the common skin manifestations of clinically latent HIV consist of cutaneous disorders normally seen in immunocompetent individuals, such as seborrheic dermatitis, psoriasis, drug hypersensitivity reactions, skin cancers, and human papillomavirus infections. In addition, increasing availability of ART has brought with it an increase in the prevalence of cutaneous side effects of antiretrovirals.
(See Chapter 22).
Seborrheic dermatitis is one of the most common dermatologic manifestations of HIV disease, affecting as many as 83% of HIV-infected individuals during the course of their disease.34 Seborrheic dermatitis may occur during all stages of HIV disease, and frequently occurs early in HIV-infection (CD4+ T cell count >500/μL).15 As is the case in immunocompetent adults, HIV-infected individuals with seborrheic dermatitis typically present with erythema and greasy scale involving the scalp, eyebrows, nasolabial folds, and posterior auricular regions. However, more disseminated forms of seborrheic dermatitis are often seen in advanced HIV disease. The forehead and malar areas, as well as the chest, back, axillae, and groin may be involved. In fact, erythroderma arising from seborrheic dermatitis may be an initial presenting sign of HIV infection in developing countries. Treatments commonly include low-potency topical steroids and topical antifungals, though more widespread forms tend to be more refractory to standard therapy.
(See Chapter 18).
Although psoriasis may develop at any stage of HIV infection, the severity of psoriasis tends to correlate with worsening immune function. For some individuals, psoriasis may be the initial presenting symptom of HIV infection, and new onset psoriasis in an individual at risk for HIV is an indication for HIV testing (Box 198-3). All clinical subtypes of psoriasis are observed in HIV-infected individuals, though guttate, inverse and erythrodermic psoriasis are the most common.35 There are no randomized trials evaluating treatments for psoriasis in HIV-infected individuals. However, based on case reports and case series, topical therapies (such as calcipotriol, corticosteroids, and tazarotene) have been recommended as first-line therapy for mild to moderate psoriasis.35 For moderate to severe psoriasis, phototherapy and ART have been recommended as first-line agents, whereas oral retinoids such as acitretin have been suggested as second-line treatment.35,36 For severe and refractory disease, methotrexate, etanercept, and infliximab have been effective, but are associated with a higher risk of opportunistic infection.35
Highly Indicative of HIV Infection (correlated with risk factors for HIV infection) Exanthem of acute retroviral syndrome Proximal subungual onychomycosis Herpetic ulcers of >1 month’s duration Oral hairy leukoplakia Kaposi’s sarcoma Eosinophilic folliculitis Multiple facial molluscum contagiosum in an adult Papular pruritic eruption of HIV Strongly Associated with HIV Infection (correlated with risk factors for HIV infection) Any sexually transmitted disease (suggestive of “unsafe” sexual practices) Herpes zoster Signs of injecting drug use Candidiasis (oropharyngeal or recurrent vulvovaginal) May be Associated with HIV Infection (correlated with risk factors for HIV infection) Generalized lymphadenopathy Seborrheic dermatitis (extensive, refractory to therapy) New Onset Psoriasis B |
(See Chapter 196).
Although the incidence of opportunistic infections in general has declined markedly since the introduction of ART, the incidence of human papillomavirus (HPV) infections has not significantly decreased and may actually have increased.37–39 HPV infections are commonly seen at all stages of HIV disease, and anogenital and oral HPV infections have been reported to occur at a higher rate in HIV-infected individuals compared to that in the general population.40,41
As immunodeficiency progresses, common warts may become larger, more numerous, confluent, and more refractory to treatment. HPV-5 can cause an unusual pattern of extensive verruca plana and pityriasis (tinea) versicolor-like lesions, similar to that seen in epidermodysplasia verruciformis.42
Clinically, anogenital warts appear similar to those seen in immunocompetent individuals; however, condyloma may be more numerous or extensive, and are often less responsive to therapy. As in immunocompetent individuals, anogenital warts in HIV-infected individuals most commonly result from infection by HPV-6 and HPV-11. Although anogenital warts are commonly considered to be benign lesions, anogenital warts in HIV-infected individuals are more likely to reflect infection with multiple HPV types, including high-risk oncogenic types -16, -18, -31, -51, -53, -56, and -58 as well as low-risk types -6 and -11.43 One study found that the majority of anogenital warts in HIV-infected individuals represent coinfection by both low-risk and high-risk HPV types.44 In another series, histologic evidence of low-grade anal intraepithelial neoplasia was found in about 50% of apparently benign anogenital warts in HIV-infected individuals.45
Management of external anogenital HPV infection in HIV-infected individuals is directed toward identifying high-grade dysplasia before progression to invasive squamous cell carcinoma (SCC). All HIV-infected individuals should be examined annually for evidence of HPV infection, especially those with a history of HPV infections. Application of 5% acetic acid may help to highlight subclinical dysplastic lesions. Based on the principles of cervical cytology, periodic anal cytology testing (via Papanicolaou smears) every 1–2 years has been proposed as a means of early detection of anal cancer.46 The sensitivity of anal cytology has been reported to range from 69%–93%, while specificity ranges from 32% to 59%.47 However, both sensitivity and specificity are reported to be higher for identification of disease within the anal canal compared to that of the external anogenital area, likely because of lower yield of cells from hyperkeratotic surfaces.48 High-resolution anoscopy/coloposcopy with biopsy should be performed in all patients with abnormal cytology. Individuals with documented external anogenital dysplasia should be followed by periodic examinations every 4–6 months, with biopsy of any new lesions at those sites.
HPV-induced oropharyngeal lesions typically present as pink or white verrucous papules, resembling anogenital condyloma. If lesions are extensive, they may coalesce to form multiple large plaques, which may transform to verrucous carcinoma (oral florid papillomatosis). Oral HPV infection has also been associated with a subset of oropharyngeal SCC, which sometimes arises from the base of the tongue and tonsils.49,50 Interestingly, advanced markers of HIV disease such as low CD4+ T cell count have not been shown to be predictive of oral HPV infection.51 Electrosurgery is effective for treatment of oral HPV-induced lesions.
The risk of HPV-induced dysplasia and malignancy is significantly higher in HIV-infected individuals compared to that in the general population. One large prospective cohort study estimated that the incidence of anal SCC in HIV-infected individuals was 42.9 times that in the general population, whereas the incidence of cervical cancer in HIV-infected women was 12.2 times that in the general population.52 A low CD4+ T cell count has been associated with a significantly increased risk of anal SCC in men, but not cervical or vulvar SCC in women.53 The effect of ART on the incidence of HPV-induced in situ and invasive SCC varies. The introduction of ART has been associated with an increase in the incidence of anal cancer in HIV-infected individuals; however, ART has also been associated with decreased incidence of cervical cancer.52
Anogenital dysplasia has been successfully treated with ablative therapies, such as surgery, electrocautery, laser, and infrared coagulation. Topical therapy is another approach, and options include imiquimod, podophyllotoxin, 80% trichloroacetic acid, and liquid nitrogen. In one small study of HIV-infected men with anal intraepithelial neoplasia, subjects were treated with imiquimod cream three times a week for 16 weeks. Nearly 80% of subjects showed complete clearance of lesions at the end of therapy.54 However, 26% of individuals who had completely cleared after imiquimod therapy experienced a recurrence after a mean follow up of 30 months.55 For minimally invasive SCC arising on the anal verge (nonkeratinized to keratinized epithelium) or on external anogenital sites (such as the penis, vulva, and perineum), surgical excision with adequate margins is recommended. Invasive SCC of the anus (transformation zone) is generally treated by radiation therapy and chemotherapy.
Staphylococcus aureus is a common bacterial pathogen causing cutaneous and systemic infections at all stages of HIV disease. No unique staphylococcal infections occur in HIV disease. Rather, HIV-infected individuals tend to present a wide range of primary skin and soft tissue infections that are normally seen in immunocompetent individuals (see Chapters 176, 178, 179, 180, 181). Secondary infection of underlying dermatoses, particularly in those colonized with S. aureus, is frequently seen in individuals with atopic dermatitis. Secondary infection of herpetic ulcers and molluscum may occur (see Chapters 193, 195).
Risk factors for staphylococcal infection in HIV-infected individuals include nasal carriage of S. aureus, the presence of an indwelling vascular catheter, and a CD4+ T cell count <100/μL.56 Interestingly, a prevalence of S. aureus carriage in the nares of 30%–50% has been documented in both symptomatic and asymptomatic HIV-seropositive individuals.56,57
More recently, an increase in methicillin-resistant S. aureus (MRSA) strains has been noted in the United States and Europe, particularly those acquired from the community.58,59 Risk factors associated with MRSA skin infections include recent hospitalization, receipt of systemic antibiotics within the past 6 months, history of injection drug use, high-risk sexual practices, and previous incarceration.58,60 The prevalence of MRSA infections does not appear to be correlated with CD4+ T cell count or viral load.60
Management of staphylococcal infections should be directed at treatment of the acute infection, treatment of any underlying dermatoses, eradication of nasal carriage of S. aureus with mupirocin ointment, and chronic oral prophylaxis for recurrent infections (See Chapter 176). Given the current prevalence of MRSA infection, culture and sensitivities of lesions and the nares are imperative.
(See Chapter 193).
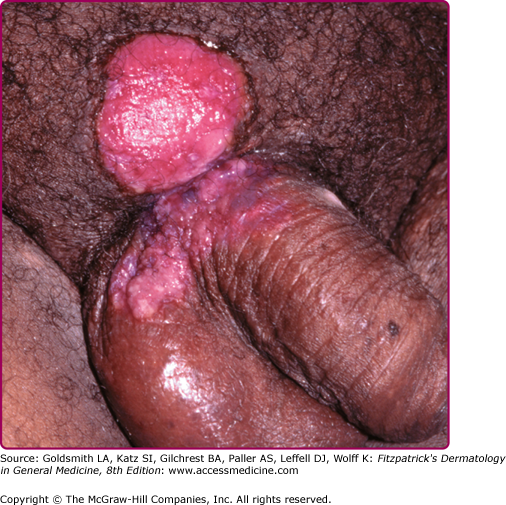
Herpes simplex virus (HSV) infection is commonly associated with HIV disease. Chronic herpetic ulcers of greater than 1 months’ duration are an AIDS-defining condition. HSV infection in HIV disease may present with severe, painful ulcerations of the perioral region, anogenital region, and digits (Fig. 198-3). Atypical morphologies, such as hyperkeratotic, verrucous papules and nodules, are sometimes observed in advanced HIV disease.61 In more advanced HIV disease, lesions typically respond less promptly to oral antiviral therapy and recur more frequently.
Foscarnet and cidofovir may be administered intravenously for infections caused by acyclovir-resistant HSV.62 Cidofovir gel has been effective as a topical therapy of acyclovir-resistant HSV infections.63 Imiquimod 5% cream is also an effective topical treatment for cutaneous herpetic infections, including those caused by acyclovir-resistant HSV strains.64
(See Chapter 194).
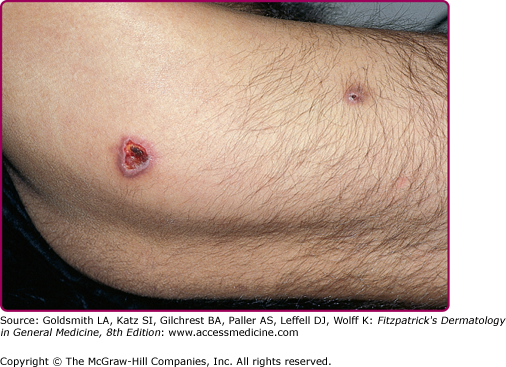
In HIV-infected individuals, varicella zoster virus infection (VZV) may present as severe varicella (primary VZV infection), persistent varicella, dermatomal herpes zoster (see eFig. 198-3.1), disseminated herpes zoster, and chronic or recurrent herpes zoster.65 In advanced HIV disease, herpes zoster may present atypically. Disseminated herpes zoster infection may manifest with scattered vesicles in the absence of dermatomal lesions. Persistent ecthymatous ulcerations and verrucous papules in the absence of a vesicular stage have also been described.66,67 Management of HIV-infected individuals with primary VZV infection should include evaluation for visceral disease. If evidence of visceral involvement is present, treatment with intravenous acyclovir (10 mg/kg every 8 hours, renally adjusted) should be initiated.68 Management of herpes zoster is based on extent of disease and degree of immunocompromise of the patient. Individuals with early HIV disease and localized cutaneous involvement may be treated with oral acyclovir, valacyclovir, or famciclovir for 7–10 days.68,69 Those with advanced HIV disease (CD4+ T cell count <200/μL), disseminated disease, visceral disease, or ocular involvement should be treated with intravenous acyclovir.68 Acyclovir-resistant VZV is rare.70
Given that the vast majority of HIV infections are sexually transmitted, individuals with HIV infection should also be screened for other sexually transmitted diseases such as Chlamydia, gonorrhea, and genital ulcerative diseases (e.g., syphilis, herpes, and chancroid).
(See Chapter 41.)
The incidence of adverse cutaneous drug eruptions is estimated to be as much as 100 times more common in individuals with untreated HIV disease compared to that in the general population, and may become more frequent with advancing immunodeficiency.71 Morbilliform eruptions are by far the most common manifestation, accounting for about 75%–95% of cases (see eFig. 198-3.2).72,73 Urticaria, erythema multiforme major, erythema multiforme minor, lichenoid eruption, vasculitis, and fixed drug eruption are also reported, though far less frequently.72,73 About 20% of cases are associated with systemic symptoms, such as fever, headache, myalgias, and arthralgias.72 Severe bullous eruptions appear to be more common in HIV disease. In one study of toxic epidermal necrosis (TEN) patients over a 6-year period, the incidence of TEN in HIV-infected individuals was found to be 375 times greater than that in the general population.74 TEN was more common in patients with advanced HIV disease and was associated with a 21% mortality rate.74
Sulfonamide drugs and penicillins are common causative agents of cutaneous drug eruptions, accounting for 75% of cases in one study.72 Factors that have been associated with increased risk of drug eruptions include female gender, CD4+ T cell count <200/μL, CD8+ T cell count >460/μL, and a history of having had drug eruptions in the past.72,75,76 In addition, individuals who are sulfa-allergic are at increased risk of more severe reactions to sulfa drugs in the setting of immune reconstitution following initiation of ART.
Management of a cutaneous drug eruption should include discontinuation of all potentially causative drugs if possible. In the absence of mucosal involvement and systemic symptoms, a causative medication may be continued with close clinical monitoring. However, if early symptoms of urticaria/angioedema or Stevens–Johnson syndrome/TEN are present, the drug should be immediately discontinued. Oral corticosteroid therapy has been reported to be helpful in decreasing the risk of cutaneous drug eruptions, and short-term corticosteroid therapy appears to be safe in most HIV-infected individuals.75,77 One placebo-controlled trial randomized 41 asymptomatic HIV-infected individuals (median CD4+ T cell count 131/μL, all but one was undergoing treatment with ART) to 8 weeks of oral prednisolone (0.5 mg/kg) or placebo to examine the immunologic consequences of corticosteroid use in HIV disease. After 8 weeks of therapy, no major side effects or HIV disease-related events had occurred, and HIV RNA levels remained stable.78 Desensitization is an option for individuals with a history of uncomplicated cutaneous drug eruptions, and has been successfully accomplished in individuals allergic to trimethoprim-sulfamethoxazole (TMP-SMZ).79
There are currently six classes of antiretroviral medications: (1) non-nucleoside reverse transcriptase inhibitors (NNRTIs), (2) protease inhibitors, (3) nucleoside reverse transcriptase inhibitors (NRTI), (4) integrase inhibitors, (5) chemokine receptor 5 antagonists, and (6) entry inhibitors (see also Chapter 231). These medications are associated with a variety of cutaneous adverse effects, including hypersensitivity reactions, lipodystrophy, retinoid-like effects, hyperpigmentation, nail changes, and injection site reactions (Table 198-4).
Drug | Mechanism | Nonmucocutaneous Side Effects | Mucocutaneous Side Effects |
|---|---|---|---|
Nucleoside Reverse Transcriptase Inhibitors | |||
Abacavir (ABC) Didanosine (ddI) Emtricitabine (FTC) Lamivudine (3TC) Stavudine (d4T) Tenofovir (TDF) Zidovudine (AZT) Zalcitabine (ddC) | Nucleoside analogs that act by incorporating themselves into the growing viral DNA chain, which eventually induces termination of viral DNA elongation. |
|
|