Fig. 24.1
Microscopy of a population of mature adipocytes
24.3 Therapeutic Uses of Autologous Fat and Stem Cell Transfer
Autologous fat grafting is used for aesthetic soft tissue volume replacement and for reconstruction of soft tissue defects. Yoshimura et al. revealed that the addition of stem cells to fat prior to transplantation, termed cell-assisted lipotransfer (CAL), has improved fat survival during autologous fat grafting (Fig. 24.2) [4]. The therapeutic uses for CAL include all areas of soft tissue volume replacement that one may consider standard autologous fat transfer:
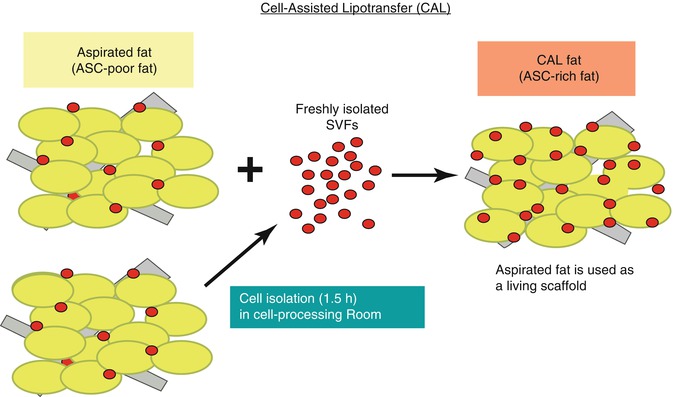

Fig. 24.2
Cell-assisted lipotransfer converts ASC-poor aspirated fat to ASC-rich fat, which increases the concentration of ASCs by 43 %, improving fat graft survival (From Yoshimura et al. [4])
1.
3.
6.
Gluteal reshaping and augmentation (Fig. 24.5) [15–17]
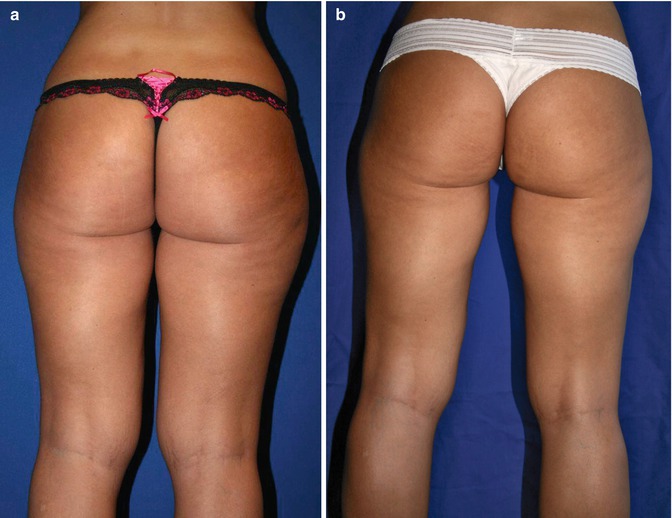

Fig. 24.5
(a) Preoperative 29-year-old woman, 67 in., 125 lb. (b) Twelve months postoperative following 650 mL total liposuction fat removal from inner and outer thigh, lower back, abdomen and flanks, gluteal sculpting, and augmentation using autologous fat transfer with 350 mL total volume replacement to central and medial buttock area (Courtesy of Robert J Troell, MD, FACS)
8.
Liposuction indentation repair
9.
Cellulite indentation repair
24.4 Stem Cell Lipotransfer: Surgical Steps
Autologous fat and stem cell grafting to any face or body part is performed with similar steps (Fig. 24.6):
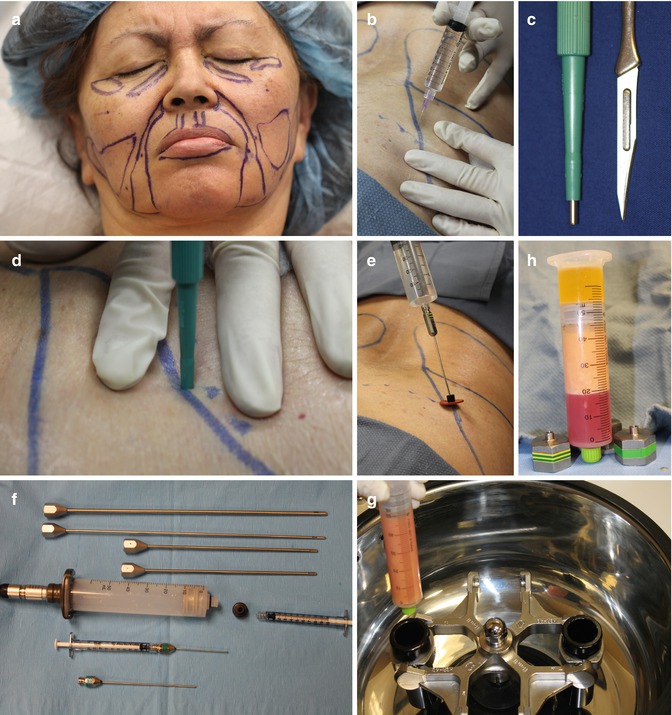
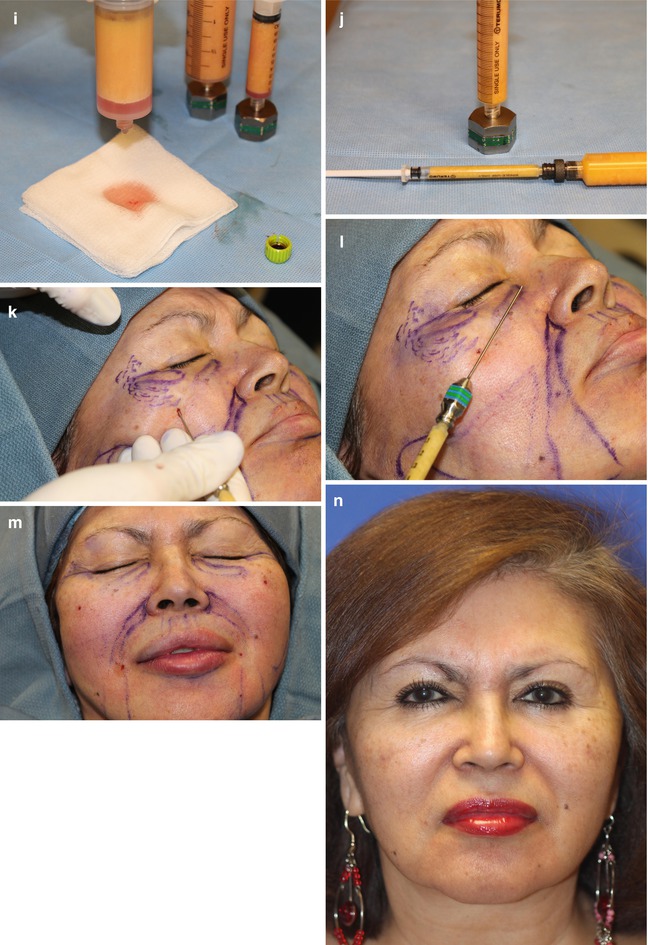


Fig. 24.6
Autologous facial fat grafting procedural steps: (a) Patient marking. (b) Local anesthesia injection. (c) Incision options (#11 knife blade v. dermal punch). (d) Incision with skin tension. (e) Wetting solution administration. (f) Fat-harvesting instrumentation using LipoKit (MediKan). (g) Closed-syringe harvesting method with suction. (h) Syringe post-centrifugation (3,500 rpms for 4 min). (i) Infranatant removal. (j) Transfer to fat injection syringe. (k) One mm punch for facial incision and supraperiosteal dissection in tear trough area. (l) Subcutaneous injection throughout face (except tear trough). (m) Immediate posttreatment appearance. (n) Four months post-procedure (Courtesy of Robert J Troell, MD, FACS)
1.
Anesthesia: general anesthesia, oral sedation with or without intravenous sedation. Facial fat grafting may also use trigeminal sensory nerve blocks.
2.
Anatomical donor site and recipient site skin marking.
3.
Prepping and sterile draping.
4.
Superwet or tumescent technique of wetting solution administration. Options include 60 mL syringe manual technique or powered infiltrators (preferred).
5.
Ultrasound energy delivery, VASER mode at 60 % (if desired).
6.
Fat harvesting (closed fat collection container or harvesting syringe).
7.
Fat processing (washing, filtering, and/or centrifugation).
8.
Stem cell isolation and purification from harvested fat (steps dependent on processing system employed).
9.
Combining fat cells with stem cells, also possibly, adding platelet-rich plasma (PRP).
10.
Fat graft (combined with stem cell and/or PRP) injection.
11.
Dressing and compression garment placement.
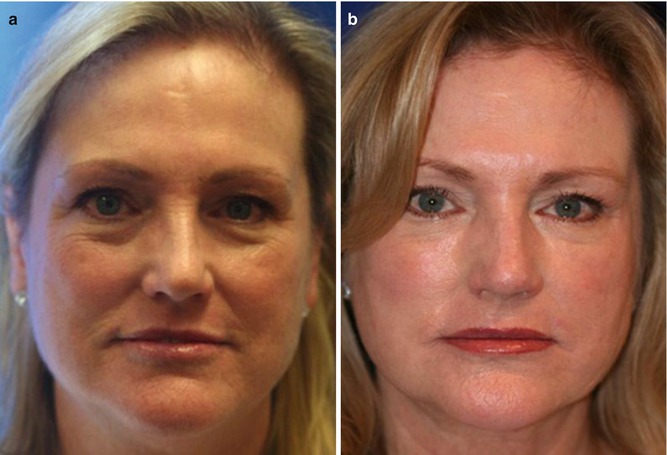
The discussion of autologous fat transfer to all areas of the body is outside the scope of this chapter. However, since there has been some controversy with fat transfer to the breast and that this has been one of the main areas of clinical research with cell-assisted lipotransfer, a more detailed review will be presented.
24.5 Stem Cell Use in Natural Breast Augmentation
The area of natural breast augmentation using autologous fat transfer, with or without the addition of stem cells, was certainly delayed by the American Society of Plastic Surgery (ASPS) policy statements: [21]
1987: “The committee is unanimous in deploring the use of autologous fat injection in breast augmentation.”
1992: “furthermore, women who seek the procedure for breast enlargement are sometimes not informed that much of the injected fat will die, causing scar tissue and calcifications…In a worst case scenario may force exploratory surgery.”
2007: “Patients considering breast augmentation need to know that fat grafting for this indication is not recommended at this time.”
2009: Investigative committee panel on breast fat grafting – “further studies and consideration warranted.”
2012: “autologous fat grafting is an effective option in breast reconstruction following mastectomy while demonstrating moderate to significant aesthetic improvement.”
These policy statements on autologous fat transfer to the breast had some scientific basis, however, in my humble opinion, seemed to be more based on fear of competition from other specialties performing natural breast augmentation versus breast implant placement, where plastic surgeons have dominance. For instance, the most recent policy statement does not mention the indication of natural breast augmentation solely for an aesthetic standpoint in woman desiring to avoid breast implant surgery with its known risk profile. The medical literature and the author’s personal experience regarding procedural risks and revision rates are far greater with breast implant surgery compared to autologous fat transfer. Coleman assisted in lobbying for the use of autologous fat grafting for aesthetic breast augmentation and has published his own experience on the subject [8].
The cause of the breast autologous fat grafting controversy was due to the lower than optimal fat survival rates observed during grafting (fat survival Bircoll, 50 %; Alexander, 65 %; Fulton, 73 %; Ueberreiter, 76 %; Khouri, 90 %). Early breast autologous fat transfer studies resulted in the formation of microcalcifications from fat necrosis (Bircoll, 1.4 %; Miller, 12.5 %; Mitnick, 24.3 %; Khouri 20 %). With improvements in cell transfer viability, the incidence of microcalcifications has diminished.
Additionally, radiologists today can clearly differentiate neoplastic radiologic properties from fat necrosis. The natural breast augmentation technique nearly always places the fat around the breast mound and not into the breast glandular tissue. The multiple views of a mammogram and the 3-dimensional views of breast magnetic resonance imaging make the diagnosis more accurate. Additionally, the improvement in fat viability also has reduced the incidence in microcalcifications.
Rubin et al. [22] compared mammographic changes following fat grafting to the breast with changes seen after breast reduction. Women were treated with fat grafting to the breast (n = 27), including admixing of autologous adipose stem cells with the fat graft, for cosmetic augmentation. Additionally, postsurgical mammograms from reduction mammaplasty patients (n = 23) were compared. Mammograms were performed 12 months after surgery. Eight academic breast-imaging radiologists reviewed each mammogram in a blinded fashion.
Differences in interpretation among individual radiologists were consistently observed; however, differences in abnormality rates were not significant for oil cysts, benign calcifications, and calcifications warranting biopsy. Scarring and masses requiring biopsy were more common in the breast reduction group. Breast-imaging reporting and data system scores were higher after breast reduction.
Compared with reduction mammaplasty, fat grafting to the breast produces fewer radiographic abnormalities with a more favorable breast-imaging reporting and data system score and less aggressive follow-up recommendations by trained and experienced breast radiologists [22]. The results of this study reveal that fat cell transfer to the breast, with or without stem cells, has little risk of causing confusion during mammogram surveillance. It is a good idea to recommend patients seeking natural breast augmentation, and breast implant surgery for that matter, to undergo a preoperative baseline mammogram.
Since fat transfer has been confirmed not to interfere with standard mammogram cancer surveillance, one can concentrate on the outcomes of fat along with stem cell use in cosmetic breast augmentation as well as in breast defect and cancer reconstruction.
A study was designed to evaluate the safety and efficacy of stem cells in breast reconstruction following partial mastectomy and radiation therapy [23]. Twenty-one women were treated with stem cells at least 12 months after radiation therapy was completed. The study concluded the following:
1.
The procedure was safe and well tolerated.
2.
There was no rejection or adverse immune response.
3.
High patient satisfaction rate (79 %).
4.
Statistically significant increase in average breast tissue thickness.
5.
No change in the breast tissue thickness from 1 month to the end of follow-up.
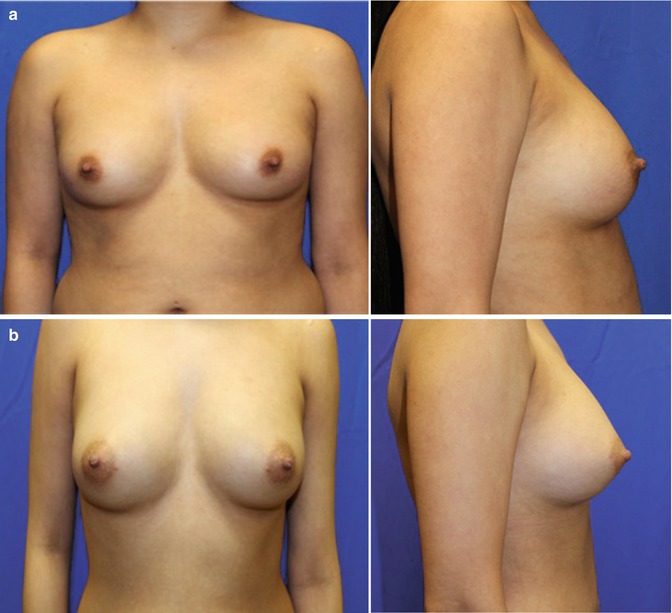
The first prospective, multicenter clinical trial (RESTORE 2) [24] evaluated ASC enhanced fat used for breast defect reconstruction in patients with T2NoMo invasive breast carcinoma after undergoing breast conservation therapy with or without radiation therapy who were in remission for at least 12 months. Seven clinical sites participated within the European Union including Spain (2), United Kingdom (2), Belgium (2), and Italy (1) with 71 patients treated with ≤150 mL of fat for their breast defects.
Fat harvesting was performed with a syringe collection technique and processed using a closed automated system isolating ASCs and then augmenting using the CAL injection technique. One fraction of the lipoaspirate was added to the Celution® system (Cytori Therapeutics; San Diego, CA) to make the ASC-enriched fat graft. Breast injections were performed using the Celbrush® (Cytori Therapeutics; San Diego, CA), which consists of a stainless steel thumb-controlled syringe adapter designed to provide microdroplet dispersion of graft. Twenty-four patients elected to undergo a second fat and stem cell transfer to the defect area after a 6-month follow-up.
The outcome measures were patient and physician satisfaction, magnetic resonance imaging (MRI) volumetric increase in breast tissue thickness, and an evaluation of side effects/complications. The RESTORE 2 study investigators reported satisfaction with 57 out of 67 patients [24], and 54 out of the 65 patients reported improvement in the breast contour based on blinded MRI anatomical assessment. There were no reported local cancer recurrences and no serious adverse events associated with the ASC-enriched fat transfer procedure; however, cysts were noted in ten patients. This prospective trial demonstrated the safety and efficacy of the treatment of breast cancer conservative treatment defects utilizing ASC-enriched fat transfer.
The availability of tissue banks for the storage of fat and stem cells may change the future of soft tissue augmentation with a transition to ASCs to be used alone as the primary method and/or used for “touch-up” soft tissue volume replacement. The use of cryopreservatives, controlled rates of freezing, and storage in liquid nitrogen has been confirmed to maintain adipocyte and ASC viability [25]. However, most storage banks recommend using the stored adipocytes within 1 year of harvesting, while there appears to be no limit on stem cells.
ASCs have valuable qualities that make ASCs an important avenue for long-term engraftment: a robust cellular nature, the ability for adipogenic differentiation (Fig. 24.7), the ability to differentiate into vascular endothelial and probably vascular mural cells, and the ease to grow and expand extensively in culture. Because of these qualities, current methods of fat transfer for soft tissue augmentation may transition to the combining ASCs in most fat transfer cases. The regenerative cells in adipose tissue are so abundant that the need for culture expansion to reach a therapeutic dose is not necessary; however, stem cells still need to be harvested, processed, and concentrated from the lipoaspirate [26].

Fig. 24.7
Differentiation of a stem cell to adipocyte
ASCs can differentiate into adipocytes in vitro; the younger adipocytes are smaller during their first 2 months after differentiation than mature cells and are more robust, which may allow less traumatic implantation through smaller cannulas. Using this tissue engineering approach for fat transfer may heighten the reliability of grafts with improved aesthetic appearance and lowering the incidence of touch-up procedures.
And with the addition of platelet-rich plasma, ASCs have the flexibility to be injected into the dermis for a combined synergy in skin rejuvenation. If microdermabrasion and dermal microneedling are performed initially, the rejuvenation treatment could be done with only the topical application of ASCs and PRP, saving the patient injections.
A joint task force of the American Society for Aesthetic Plastic Surgery (ASAPS) and the American Society of Plastic Surgeons (ASPS) formatted a position statement on the use of “stem cells” in aesthetic surgery [27]. This policy statement was based on a systematic review of the peer-reviewed literature and concluded that “while there is tremendous potential for the future use of stem cells in aesthetic surgical procedures, the scientific evidence and other data are very limited in terms of assessing the safety or efficacy of stem cell therapies in aesthetic medicine.” The remaining part of this manuscript will touch on only a fraction of the current peer-reviewed journal articles published in the medical literature regarding the use of stem cells and their mechanism of action in a multitude of fields of medicine.
Based on the current state of knowledge, the task force made the following recommendations to ASAPS/ASPS members and their patients:
Terms such as “stem cell therapy” or “stem cell procedure” should be reserved to describe those treatments or techniques where the collection, concentration, manipulation, and therapeutic action of the stem cells are the primary goal, rather than a passive result, of the treatment. For example, standard fat grafting procedures that do transfer some stem cells naturally present within the tissue should be described as fat grafting procedures, not stem cell procedures.
The marketing and promotion of stem cell procedures in aesthetic surgery are not adequately supported by clinical evidence at this time.
While stem cell therapies have the potential to be beneficial for a variety of medical applications, a substantial body of clinical data to assess plastic surgery applications still needs to be collected. Until further evidence is available, stem cell therapies in aesthetic and reconstructive surgery should be conducted within clinical studies under Institutional Review Board approval, including compliance with all guidelines for human medical studies.
The collection and reporting of data on outcomes and safety by any physician performing stem cell therapies are strongly encouraged in order to advance the knowledge and science of stem cells.
Stem cell-based procedures should be performed in compliance with FDA regulatory guidelines. If devices are employed that are subject to regulation by the FDA, surgeons should use these devices with appropriate approval in place, especially when used for investigational purposes.
Patients are advised to seek consultation for aesthetic procedures by a surgeon certified by the American Board of Plastic Surgery. These physicians are able to properly evaluate a patient’s concerns and offer a wide range of safe solutions. Extreme caution should be exercised when a physician is promising results from any treatment that sound too good to be true.
These recommendations are from two societies and are not considered dictum. Physicians promoting stem cell augmented autologous fat transfer or stem cell administration alone, many wish to follow some or all of these recommendations. Scientific laboratory and clinical research will guide the future of therapeutic indications for stem cell use.
24.6 Therapeutic Uses of Stem Cell
A number of clinical trials using freshly isolated or cultured ASCs are ongoing in more than ten countries, most in Europe and Asia.
The therapeutic uses of stem cells include:
7.
Radiation necrosis
8.
Wrinkle treatment
10.
Liver insufficiency
11.
Nonalcoholic chronic liver disease
12.
Stress urinary incontinence
13.
Renal ischemia
24.7 Stem Cell Use in Orthopedic Surgery
In the 1990s, stem cells were combined in processes for the repair of cartilage, bone, tendon, muscle, and other connective tissues. The field of stem cells in orthopedic surgery has expanded to nearly every aspect of the specialty.
Bone matrix (BM) is an acellular, cross-linked cancellous bone graft, often porcine derived. Because it is a biologic tissue, it has advantages over synthetic materials. Arca et al. evaluated the potential for BM to support the growth and differentiation of human mesenchymal stem cells (ASCs) in vitro [31]. Long-term growth analysis by confocal imaging and histology demonstrated ASCs survived on the bone matrix scaffold in culture. The authors conclusion was that bone matrix had osteo-inductive capabilities and when supplemented with osteogenic stimulants (growth factors) supported osteogenesis by ASCs.
ASCs’ influence in fracture healing was studied in an immunodeficient rat femur nonunion fracture model [32]. Local transplantation of human ASCs in a rat femur fracture revealed both radiographic and histological evidence promoting fracture healing with significant improvement of biomechanical function at the fracture site compared with local transplantation of human fibroblasts. Histological capillary density and laser Doppler blood perfusion imaging were significantly greater in the stem cell group compared to the human fibroblast-treated group. These findings suggest stem cells augmented angiogenesis and bone tissue formation.
Rat growth factors (bone morphogenetic protein-2 (BMP-2), vascular endothelial growth factor (VEGF), and angiopoietin-1) in the tissue surrounding the fracture were most upregulated in the human ASC group. They also showed that the presence of BMP-2 or VEGF activated the proliferation and migration of ASCs in vitro. These results indicate that human ASCs interacted with resident cells stronger than other cells and promoted fracture healing more effectively. Additionally, human-specific antibody immunohistochemistry revealed direct differentiation of human ASCs into osteoblasts and endothelial cells in the newly formed rat callus. They deduced that the grafting of ASCs into fractures may become a useful strategy for cell-based bone regeneration [32].
This data revealed that growth factors stimulated stem cell function. ASCs could differentiate into vascular endothelial cells promoting increase tissue blood supply and ASCs differentiate into osteoblasts, which produce new bone formation. The bone formation requires a scaffold or bone matrix, growth factors, and stem cells themselves for this process to occur.
Atesok et al. [33] evaluated the effects of a type of stem cell, the local endothelial progenitor cell (EPC), on bone regeneration. A segmental bone defect was created in a rat femur and fixed with a mini-plate in both the stem cell and control group with the animals sacrificed at 1, 2, 3, and 10 weeks postoperatively. Healing was evaluated with radiographic, histological, and quantitative microcomputed tomography (micro-CT) scans. At 10 weeks, all the animals in the EPC-treated group had complete union, while none in the control group achieved union. Histological evaluation revealed that specimens from EPC-treated animals had abundant new bone and vessel formation compared to that in controls. Micro-CT assessment showed significantly improved parameters of bone volume, bone volume density, trabecular number, thickness, and spacing, bone surface, and bone-surface-to-bone-volume ratio in the EPC group. Atesok et al. [33] concluded that there was significantly enhanced bone regeneration with the presence of the local endothelial progenitor cells.
In a follow-up study [34], the same group evaluated the effects of the local endothelial progenitor cell (EPC) on the microarchitecture and biomechanical properties of a segmental bone defect in a rat model. New bone formation was assessed with radiographs, microcomputed tomography, and biomechanical testing. They showed that stem cells significantly enhanced fracture healing with superior radiographic bone formation [34].
These studies confirm the importance of a subset of stem and progenitor cells that differentiate into blood vessels. Another subset of stem cells can differentiate into other tissues cells, such as osteoblasts, in a specific cellular environment with local cellular and growth factor influences.
24.8 Stem Cell Use in Wound Healing
Dermal wound healing is a highly coordinated process, where fibroblasts interact with surrounding cells and produce extracellular matrix (ECM), glycoproteins, and various cytokines. After tissue injury, fibroblasts migrate into the initial fibrin-based matrix and proliferate and begin the production of ECM and collagen. Wound edges reapproximate through the wounds contractile properties.
Histological features of photo-aged skin show marked alterations of ECM composition. The collagenous component of dermal ECM is responsible for the strength and resiliency of skin and is a key factor involved in sun-induced skin pathologic changes. In photo-aged human skin, the precursors of type I and III collagen are significantly reduced in the papillary dermis and their reduction has been shown to correlate with the clinical severity of photo-aging. This reduction results from a combination of reduced pro-collagen biosynthesis and increased actions of cellular collagenase, also known as matrix metalloproteinase (MMP), involved in the breakdown of extracellular matrix.
Few studies have researched the effects of ASCs on fibroblasts, which play a key role in wound healing, scar formation, and solar skin damage. Kim et al. [35] investigated the possible roles of ASCs in the skin wound-healing process, specifically related to human dermal fibroblast (HDF) activation with cellular migration, proliferation, and collagen synthesis. In addition, secretion of type I collagen was examined by measuring messenger ribonucleic acid (mRNA) levels of ECM proteins and the effect on the migration activity of HDFs was tested in an in vitro wound-healing model. They also studied the effect of ASCs on wound size and skin re-epithelialization in an animal model. The results revealed that ASCs promoted human dermal fibroblast (HDF) proliferation, not only by cell-to-cell direct contact, but also by paracrine activation through secretory factors. Interestingly, this proliferative effect was completely abolished by boiling, suggesting that it was due to a specific receptor/ligand interaction [37]. In this study, ASCs were superior to HDFs in promoting HDF proliferation and in upregulation of type I collagen secretion by fibroblasts. ASC enhanced HDF proliferation in a dose-dependent manner. ASCs enhanced the secretion of type I collagen in HDFs by regulating the mRNA levels of extracellular matrix (ECM) proteins: upregulation of collagen type I, type III, and fibronectin and downregulation of the MMP-1 collagenase enzyme. The concentrations of type I collagen and fibronectin were found to be at least 1,000-fold higher than those of other growth factors. In an animal model, ASCs significantly reduced the wound size and accelerated the re-epithelialization from the wound edge.
Kim et al. [36] noted that ASCs were found to produce various growth factors, such as platelet-derived growth factor (PDGF), insulin-like growth factor (IGF), and keratinocyte growth factor (KGF), in addition to the previously reported growth factors: fibroblast growth factor (bFGF), transforming growth factor (TGF-β), hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF). These cytokines operate in concert with other regulatory proteins. For fibroblasts, the main implicated cytokines are PDGF, TGF-β1, FGF, as well as interleukins (IL)-1β, 6, and 10. Among these, PDGF and TGF-β1 may play a key role in the wound-healing process. The conclusions of this study were that ASCs promote dermal fibroblast proliferation by cell-to-cell contact and by paracrine activation through secretory factors. ASCs enhanced the secretion of type I collagen in fibroblasts and stimulated the migration of fibroblasts. Additionally, ASCs significantly reduced the wound size accelerating re-epithelialization [35]. This data suggests that ASCs can be used for the treatment of solar-damaged skin and can be used to improve wound healing.
Amazingly, stem cells can influence adjacent cells, in this case fibroblasts, to migrate, proliferate, and secrete substances. The cellular influence is from both direct cellular contact and by growth factor secretion.
Blanton et al. [36] examined the efficacy of ASCs, when supplied either alone or along with platelet-rich plasma, to improve wound healing. A porcine full-thickness wound model was used to compare six topical treatments: platelet-poor plasma, platelet-rich plasma, autologous adipose stem cells plus platelet-poor plasma, autologous adipose stem cells plus platelet-rich plasma, allogeneic adipose stem cells containing green fluorescent protein plus platelet-poor plasma, and saline (control). There was no significant difference in the re-epithelialization rate; however, treatments containing ASCs demonstrated increased capillary densities. Wound cosmesis was improved with the combination of ASCs with platelet-rich plasma. ASC samples had approximately seven-fold higher levels of vascular endothelial growth factor. Transgenic green fluorescent protein combined with ASCs became incorporated near new blood vessels [36]. They concluded that ASCs enhanced the healing process in association with growth factors present in platelet-rich plasma. Additionally, the perivascular ASC localizations suggest a function in enhancing blood supply through providing physical and paracrine support to newly forming vessels. This study reiterates that the improved blood supply in the presence of stem cells is directly related to vascular endothelial growth factor secretion.
The inflammatory response in wound areas induces oxygen deficiency. The objectives of the study by Lee et al. [37] were as follows: (1) does hypoxia alter the wound-healing function of adipose-derived stem cells and (2) what are the factors responsible for wound healing? Hypoxia enhanced the proliferation of ASCs. The conditioned medium of ASCs harvested under hypoxic conditions significantly promoted collagen synthesis and the migration of human dermal fibroblasts, compared with the cellular milieu from a normal oxygen environment. Hypoxia upregulated angiogenic growth factors, including vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF) as determined by mRNA and protein measurements. To test the effects of the growth factors in the healing wounds in an animal model, their effects were inhibited by using neutralizing antibodies to VEGF and bFGF. This reversed the migration of human dermal fibroblasts. In summary, these study results infer that hypoxia increases the proliferation of ASCs and the hypoxia enhances the wound-healing function of ASCs by upregulating the secretion of VEGF and bFGF [37].
24.9 Stem Cells in Cardiovascular Disease
Knowledge of the mechanisms of cardiac cell regeneration is critical for the identification of medical treatment strategies aimed at restoring function and structural integrity of an acutely injured heart as with acute myocardial ischemia or a chronic failing heart as in congestive heart failure. Although a mass of research has been compiled, there is still debate on the role of stem cells from the bone marrow and in the heart itself in terms of both normal cellular regeneration as well as healing mechanisms from injury. However, most believe that the heart is no longer considered a postmitotic organ, but it’s a continually self-renewing organ. Anversa et al. [40




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree