Incidence of Phenotypic Classification
Congenital melanocytic nevi (CMN) are present at birth, or very occasionally arise in the first few years of life; tardive CMN. These lesions grow commensurate with the child, but tardive lesions can appear to grow more rapidly as the full extent of the CMN is often not initially apparent. The incidence ranges from 1% for small single CMN to approximately 1 in 20,000 for nevi projected to be greater than 20 cm in diameter in adulthood. CMN are commonly classified according to maximum projected adult size and the total number of lesions at birth. The most recently proposed classification of size is small (CMN with an estimated diameter in adulthood of less than 1.5 cm), medium (M1, 1.5–10 cm and M2, 10–20 cm), large (L1, 20–30 cm, L2, 30–40 cm) and giant (G1, 40–60 cm, G2, >60 cm). However, authors prefer not to employ the term giant as it is often unacceptable to patients and families. The estimates of projected adult size are determined in a variety of ways, none of which is accurate, but remain the most reliable methods currently available. Some clinicians use a scaling factor applied to the CMN diameter at birth. More specifically, a CMN on the head is predicted to enlarge by a factor of 1.7, on the trunk and upper extremity by a factor of 2.8, and on the lower extremity by a factor of 3.3. This approach is not without fault, as CMN often affect more than one region of the body and it does not account for significant differences in growth between the sexes. Other clinicians therefore estimate the projected adult size by drawing the child’s CMN onto an adult body map, and estimating the longest non-circumferential diameter. CMN can present as an isolated singular lesion or can be accompanied by a variable number of smaller discrete so-called satellite lesions. These lesions should be counted or estimated, as each satellite lesion represents an additional CMN.
Histological and Clinical Appearances of CMN
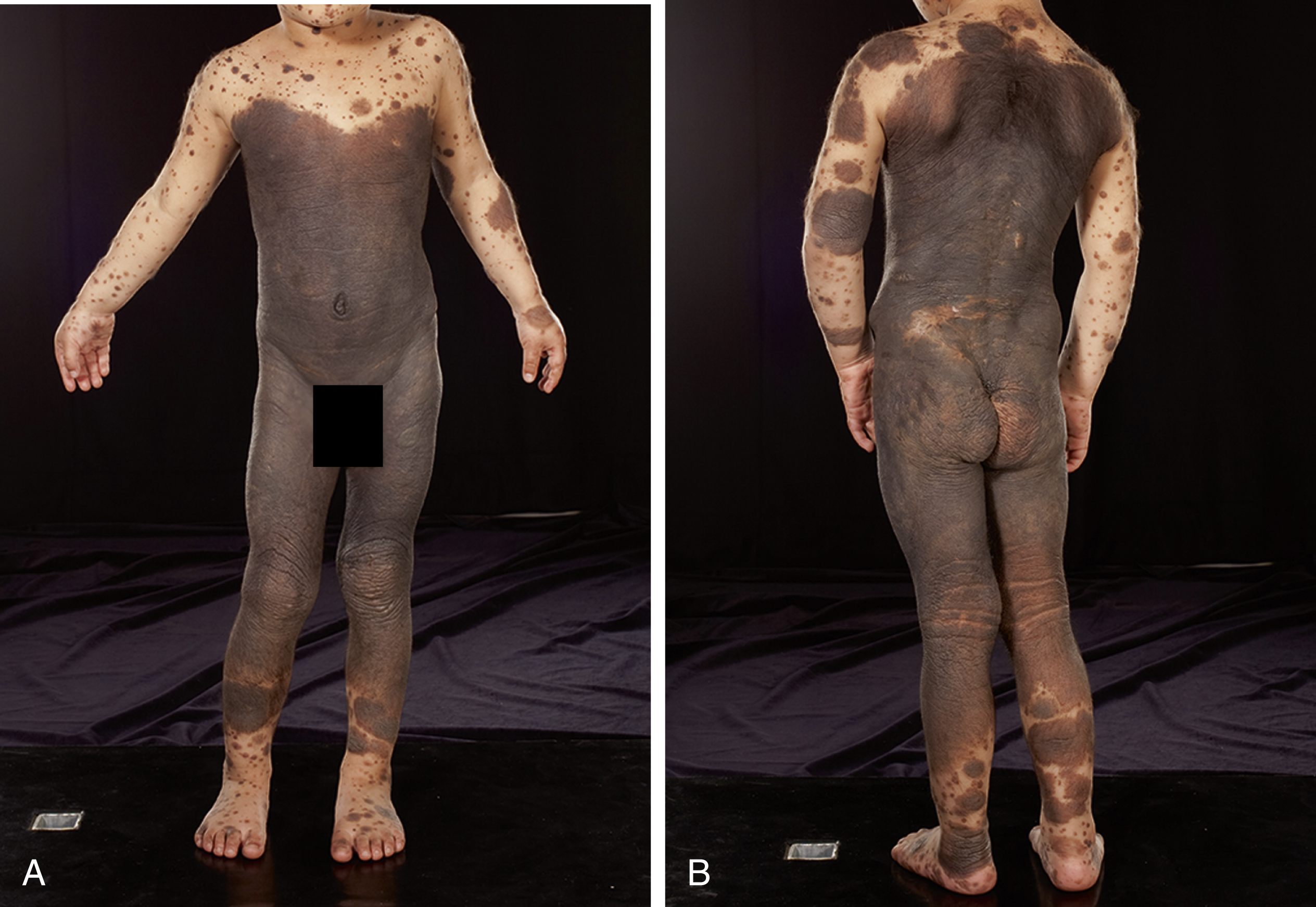
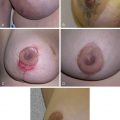
CMN consist of nests of nevus cells, which differ from normal melanocytes in their nondendritic morphology and proclivity to form nests. Melanocytic nests are present at the dermoepidermal junction, within the dermis and/or subcutaneous tissue, and nevus cells can be localized between collagen bundles and found surrounding dermal appendages, blood vessels, and nerves. Small and medium-sized CMN generally present clinically as homogenous pigmented lesions with well-delineated borders, an increase in skin surface markings, and variable hypertrichosis. Larger CMN express color variegation, are often asymmetric with irregular borders, can have a nodular surface, and are usually hypertrichotic ( Fig. 14.1 ). Nodules are often appreciated to arise within the substance of the CMN or can be present at birth. Any such focal change within a CMN should be viewed with suspicion and reviewed by a pediatric dermatologist. Although malignancy should always be considered, the vast majority of nodules are benign and repeated excisions can thus be avoided.
The most common proliferations can be divided into classic proliferative nodules and neuroid overgrowths. The classic nodules are well-defined, smooth, round or oval proliferations, are soft or firm, and occasiaonally have a shiny surface. The majority are 0.5–2 cm in diameter and often pink or less pigmented than the surrounding CMN. Although classic proliferative nodules have been suggested to be potential precursors to melanoma, definitive association is not supported in the literature. Histopathology and genetic investigations can assist in differentiating between benign proliferative nodules and melanoma, in particular array comparative genomic hybridization. In contrast, neuroid overgrowths are poorly defined, round or ovoid, soft, and often pendulous. These can be several centimeters to >20 cm in diameter, and are lighter than the surrounding CMN or pink-red. Transformation of neuroid proliferations to melanoma has not been reported.
Neurological Associations of CMN
Individuals with CMN can be divided into those with a cutaneous phenotype only (either single or multiple), and those with extracutaneous features (either neurological or facial), the latter then termed congenital melanocytic nevus (CMN) syndrome . The projected adult size of the lesion, as well as the total number of CMN at birth, is known to be associated with adverse clinical outcomes. Neurological abnormalities are the most common complication of CMN, and this association has previously been known as neurocutaneous melanosis . The latter term has now been supplanted by CMN syndrome , as the neurological complications are not always melanotic in nature, and the literature has in the past been highly confusing by including both benign congenital neurological disease and primary melanoma of the CNS under the term neurocutaneous melanosis.
The prevalence of neurological abnormalities increases with the size of the largest CMN and the total number of nevi. , CMN distribution, in particular lesions on the posterior axis, is not associated with an increased risk of neurological anomalies. The previously suggested connection between cutaneous distribution and neurological risk was confounded by the fact that the largest lesions are often located on the back. The most common neurological complication is intraparenchymal melanosis, where foci of melanin-producing cells are found within the brain parenchyma, most notably in the amygdala. Intraparenchymal melanosis without other neurological abnormalities on MRI are benign congenital lesions and asymptomatic in approximately half of cases. The other half of cases can exhibit developmental delay and/or serizures. Developmental delay varies from mild speech delay to global developmental retardation or behavioral problems, such as attention deficit hyperactivity disorder or autistic spectrum disorder. Thus, when intraparenchymal melanosis is seen, authors suggest annual neurodevelopmental monitoring until school age, in an effort to detect developmental issues early. Even when symptomatic (delay or seizures), these lesions are not a sign of malignancy or of impending death, and should not be considered for biopsy or surgical resection. Although rare, progression to melanoma remains a possibility, however, this can arise in the central nervous system (CNS) even in the absence of visible intraparenchymal melanosis.
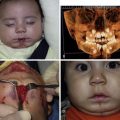
Other congenital neurological anomalies, such as Dandy–Walker malformation, hydrocephalus, and brain or spinal cord tumours, can occur, with or without leptomeningeal melanosis. These more complex neurological abnormalities are much more commonly symptomatic with manifestation of seizures and developmental delay, and therefore necessitate more rigorous management, with regular MRI, neurological assessments and, frequently, neurosurgical consultation. This smaller (<10%) cohort of patients appears to be at highest risk of melanoma. Interestingly, it has been shown that neurological symptoms in patients with CMN can be present in the absence of any perceptible radiological abnormality. , This finding has been proposed to be related to lesions that cannot be visualized with current imaging techniques. Thus, the authors’ current management protocol includes a gadolinium-enhanced MRI of the CNS (brain and whole spine) for any child with the onset of abnormal neurological signs or symptoms at any age (irrespective of a previously normal scan) and routinely for all children with two or more CMN at birth, independent of size or site, under the age of 1 year (ideally within the first 6 months of life) ( Fig. 14.2 ). Follow-up of children with CMN or CMN syndrome depends on the severity of the cutaneous phenotype, the neurological presentation, and the risk of melanoma. Patients should have access to a specialist pediatric dermatology clinic with input from a multidisciplinary team, including pediatric plastic surgeon, neuroradiologist, neurologist, neurosurgeon, psychologist, and oncologist when necessary.
Other Associated Features of CMN
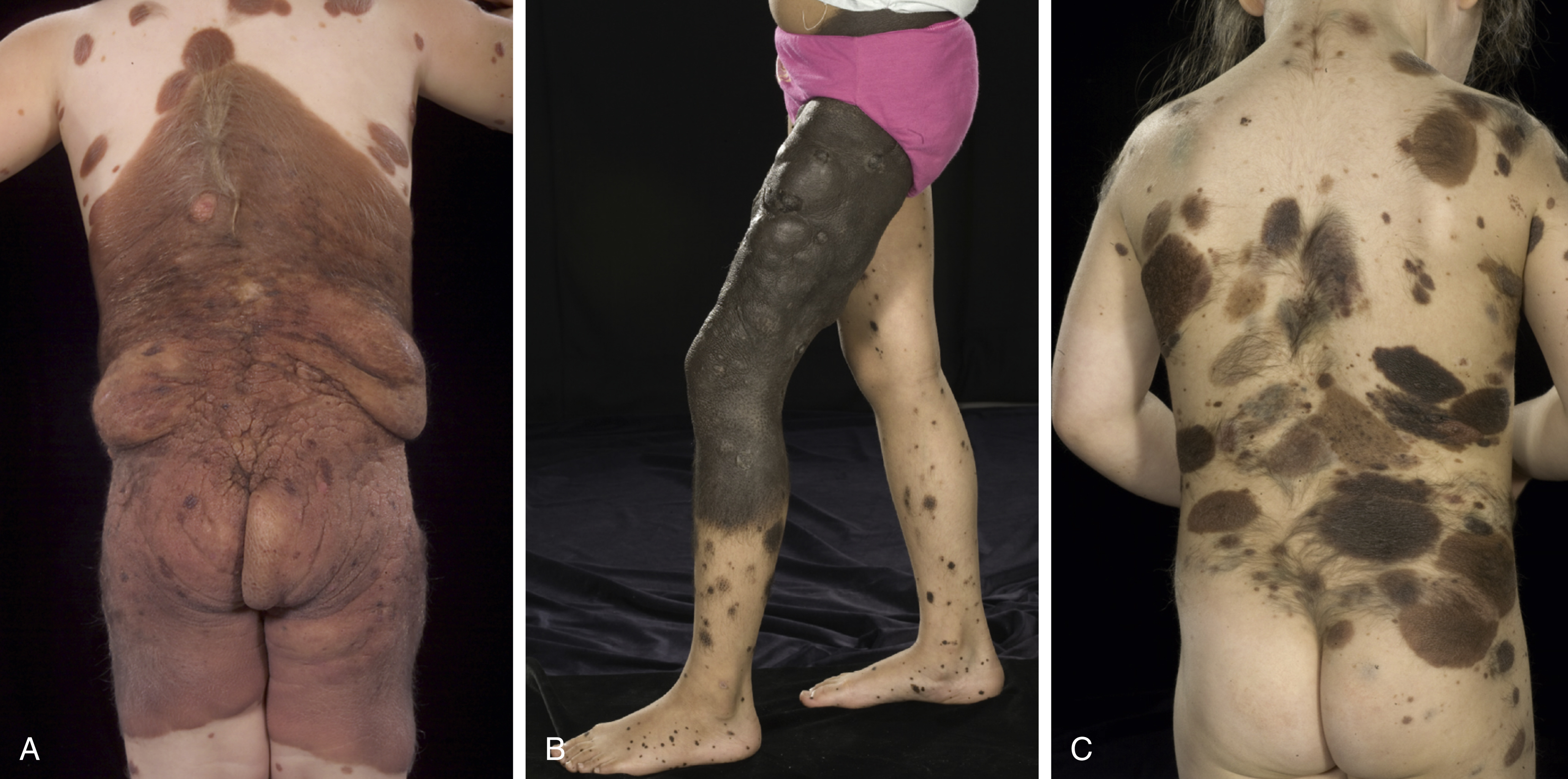
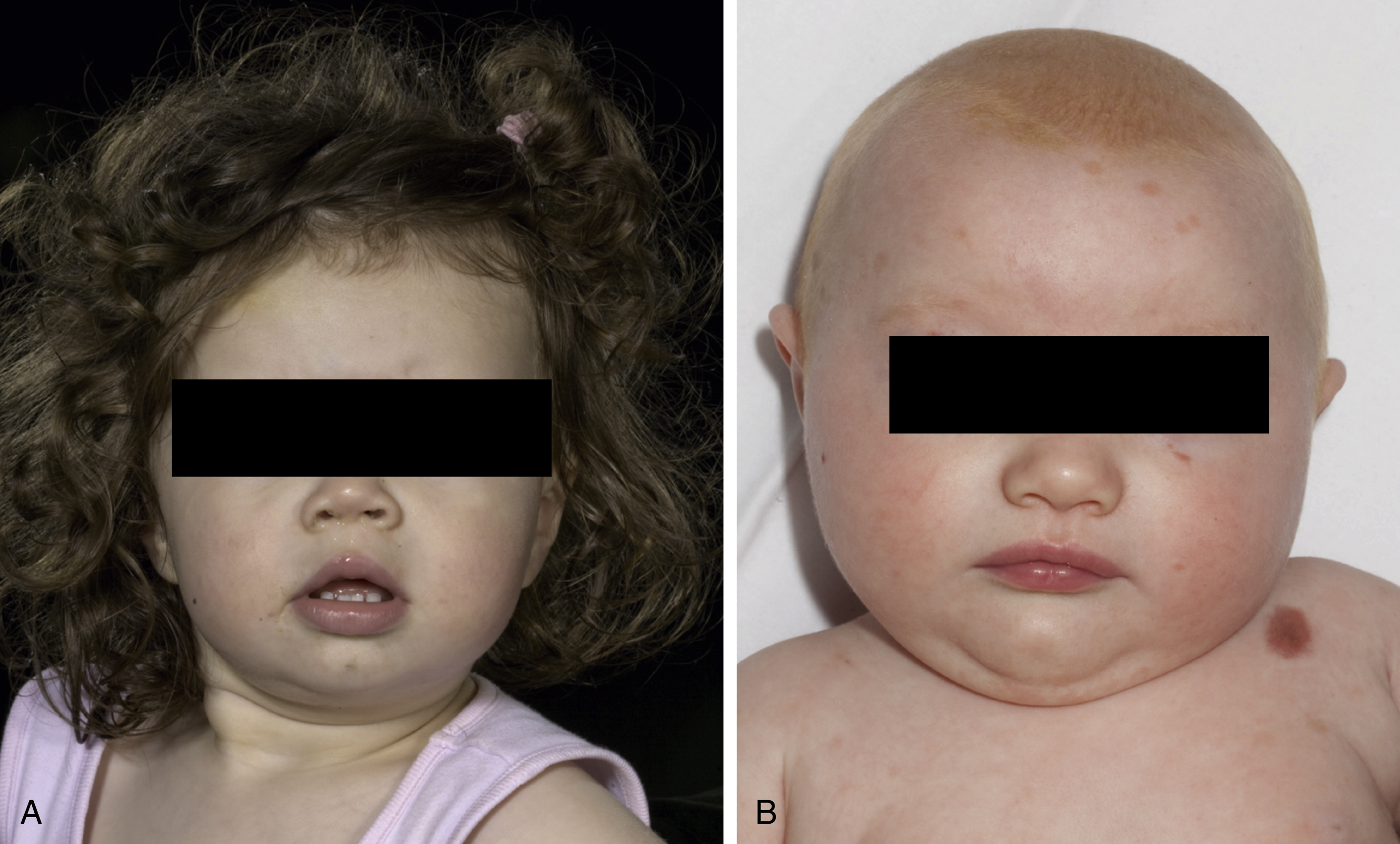
Characteristic facies in children with CMN has expanded the description of this condition ( Fig. 14.3 ). These typical features include a wide or prominent forehead, apparent hypertelorism, eyebrow variants, periorbital fullness, small/short nose, narrow nasal bridge, anteverted nares, broad nasal tip, broad or round face, full cheeks, prominent premaxilla, open mouth appearance, everted lower lip, and prominent or long philtrum ( Table 14.1 ). In support of the notion that children with CMN display a typical facies is the fact that aberration in neural crest development gives rise to melanocytic and facial development in humans. Indeed, a single genetic abnormality may be responsible for CMN, neurological abnormalities, and the typical facies of these children, by affecting the pluripotent cephalic neural crest cells that ultimately differentiate into diverse cell types, including melanocytes, neurons, glia, osteocytes, and chondrocytes. The genes on the RAS/MAPK pathway are known to be involved in facial development, and individuals with germ-line RASopathies have characteristic facies. This clinical finding is therefore consistent with NRAS mosaicism affecting neural crest or neuroectodermal structures, including pigment cells, cells of the CNS as well as bone and cartilage of the face. Furthermore, many RASopathies manifest abnormalities in growth and endocrine dysfunction, and a recent retrospective study described metabolic and hormonal disturbances in children with CMN.
| Facial Feature | Prevalence (%) |
|---|---|
| Wide or prominent forehead | 74 |
| Apparent hypertelorism | 12 |
| Eyebrow variants | 36 |
| Periorbital fullness | 27 |
| Small/short nose | 25 |
| Narrow nasal ridge | 18 |
| Flaring nares | 15 |
| Broad nasal tip | 34 |
| Broad or round face | 71 |
| Full cheeks | 55 |
| Prominent premaxilla | 10 |
| Prominent everted lower lip | 47 |
| Prominent or long philtrum | 20 |
Thus, the term congenital melanocytic nevus syndrome is used to characterize and unite these aspects of the condition. The following two criteria are included, both of which must be present: first, the presence of a CMN of greater than 5 cm projected adult size or more than one CMN of any size at birth; second, neurological involvement (clinical or radiological) and/or the presence of three or more typical facial features.
Melanoma and CMN
Primary melanoma of the skin or CNS is a rare complication of CMN in childhood. However, the incidence varies with the severity of the cutaneous phenotype. The cited estimates of the incidence of malignancy have been extremely variable, ranging from 0 to 40%. More recent and reliable data on the incidence of malignant melanoma and the clinical phenotype of CMN associated with its development have been published. Large studies have demonstrated that although there is an increased risk of melanoma in patients with CMN (especially during childhood and adolescence), as compared to the general pediatric population, it is in fact much lower than previously reported estimates, with the overall incidence for all sizes of CMN between 0.7% and 2.4%. , , However, the risk of melanoma increases with CMN of projected adult size >40 cm and accompanied by multiple smaller CMN, and has been estimated at 10–15%. , In a substantial proportion of cases, the primary melanoma develops within the CNS rather than the skin. , , A review of the literature concluded that primary CNS melanoma accounts for approximately one-third of melanoma occurring in patients with CMN. However, a recent prospective study demonstrated CNS melanoma to be more common in childhood than cutaneous melanoma. All cases were in children with multiple CMN (two or more at birth), and the majority occurred in those where either the largest CMN was >60 cm projected adult size or there were multiple CMN with no large nevus. Thus, the incidence of malignant transformation was estimated at 8% in children with CMN of projected adult size >60 cm, and 1% in children with any other cutaneous phenotype. However, recent data has revealed that the best predictor of risk for melanoma is not cutaneous phenotype, but rather the outcome of the screening MRI of the CNS, with a higher risk in children with complex congenital abnormalities of the CNS. When melanoma does arise in these children, it is usually highly aggressive, refractory to therapy and rapidly fatal. , , The median age of onset is estimated at 2 years. Cutaneous melanoma arising in CMN usually presents as a new nodule, arising primarily in the deep dermis or subcutis. Primary CNS melanoma presents as either a solid intraparenchymal tumor or more commonly as leptomeningeal melanoma and is more common than cutaneous melanoma.
Genetics of CMN and Implications for Treatment of Melanoma
The causative genetics of multiple CMN have been elucidated over the last decade. The majority (70%) of cases are caused by postzygotic mosaicism for oncogenic mutations in codon 61 of NRAS , and CNM syndrome is therefore a mosaic RASopathy (genetic disorders of the RAS/MAPK pathway). Recently, mutations in BRAF have been described as a cause of multiple CMN in 7% of cases. , These developmental mutations predispose to melanoma in individuals with CMN syndrome by acting as the first insult in the subsequent occurrence of melanoma.
Where melanoma does arise, genotyping of the tumor is relevant for therapy. The use of a MEK inhibitor (Trametinib), which targets the MAPK pathway, has been trialed on a compassionate basis for treating children with CMN melanoma associated with an underlying NRAS mutation, resulting in clear symptomatic relief but not disease control. BRAF inhibitors would theoretically be appropriate in BRAF -mutant CMN with melanoma, although no cases have been published thus far. BRAF inhibitors would, however, be contraindicated in NRAS -mutant CNS.
Analysis of copy number is known to be highly contributory to CMN management. This has been demonstrated to differentiate between benign and malignant proliferations both in the skin and more recently in the CNS. , If a suspected lesion (skin or CNS) is biopsied, some should be sent for histology, and some fresh for DNA extraction and ideally both NRAS/BRAF genotyping, and whole genome copy number (array CGH or SNP array).
Evidence is, however, suggestive of a germ line predisposition for the development of CMN, with a higher prevalence of smaller CMN in family members of affected individuals, , as well rare reports of large CMN in first-degree family members. , In one UK study, homozygosity and compound heterozygosity of variants in the melanocortin-1-receptor gene ( MC1R ) were found at a higher prevalence in individuals with CMN and certain variants were associated with a more severe cutaneous phenotype.
Surgical Management of CMN
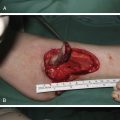
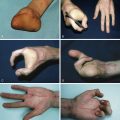
Irrespective of size, long-standing practice for treatment of CMN has been surgical removal. A commonly cited argument in favor of early surgical excision is to decrease or avert the potential for malignant degeneration to melanoma. However, surgical excision has not been shown to reduce the risk of malignant melanoma formation in patients with CMN . First, individuals at high risk for malignancy have very extensive CMN associated with numerous smaller CMN, which can total in excess of 400 CMN. Complete excision of a very large CMN and all smaller nevi remains an impossibility ( Fig. 14.4 ). Second, there is some evidence that the behavior of nevus cells may in fact be altered by surgical intervention. In one study, tissue expansion was associated with the development of new nevi, which was supported by individual case evidence from other centers (A Taieb, personal communication). The latter is thought to occur secondary to either non-nevus cell melanocytes that are activated by some aspect of treatment with tissue expanders or that treatment promotes the spread of nevus cells from the primary nevus, a type of benign metastasis known to occur in CMN and Spitz nevi. Moreover, an increased incidence of darkening of surgically treated areas of CMN and untreated areas of partially excised CMN was noted, and new CMN were found to appear at the edge of the treated area. An additional important consideration is that the occurrence of melanoma is not restricted to the CMN , and thus surgical excision of the primary CMN does not preclude development of melanoma in the CNS or other extracutaneous location.