40 Congenital melanocytic nevi
Synopsis
 Congenital melanocytic nevi (CMN) are composed of clusters of nevomelanocytes that are generally present at birth but occasionally arise as late as several years. These lesions arise from melanocytic stem cells that migrate from the neural crest to the embryonic dermis and upward into the epidermis. They may also migrate into the leptomeninges.
Congenital melanocytic nevi (CMN) are composed of clusters of nevomelanocytes that are generally present at birth but occasionally arise as late as several years. These lesions arise from melanocytic stem cells that migrate from the neural crest to the embryonic dermis and upward into the epidermis. They may also migrate into the leptomeninges.
 Although the bulk of these lesions are small and benign, some cover large portions of the body or can be in conspicuous locations, and may create an aesthetically displeasing appearance, resulting in psychological issues. Furthermore, their potential for malignant degeneration causes anxiety for the parent, primary care physician, and surgeon alike.
Although the bulk of these lesions are small and benign, some cover large portions of the body or can be in conspicuous locations, and may create an aesthetically displeasing appearance, resulting in psychological issues. Furthermore, their potential for malignant degeneration causes anxiety for the parent, primary care physician, and surgeon alike.
 Small pigmented nevi are present in 1 in 100 births, large nevi are present in only 1 in 20 000 births, and the giant lesions are even less common. As a result, most surgeons have little experience with them and little opportunity to develop a rational protocol for their treatment.
Small pigmented nevi are present in 1 in 100 births, large nevi are present in only 1 in 20 000 births, and the giant lesions are even less common. As a result, most surgeons have little experience with them and little opportunity to develop a rational protocol for their treatment.
 The goal of this chapter is to review the pathophysiology and natural history of CMN, summarize the risk of malignant degeneration, and provide a rational approach to treatment.
The goal of this chapter is to review the pathophysiology and natural history of CMN, summarize the risk of malignant degeneration, and provide a rational approach to treatment.
Introduction
• CMN consist of clusters of nevomelanocytes that develop in utero. Although many congenital nevi are visible at birth, some are “tardive,” probably because they are too small to be detected at birth or do not have sufficient melanin.1,2
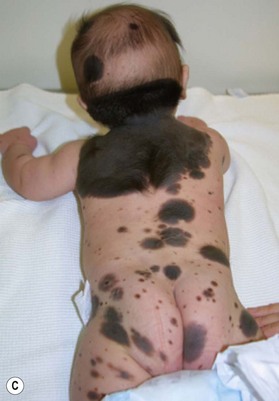
• CMN are one of the risk factors for eventual development of cutaneous and extracutaneous melanoma, with larger nevi having greater risk. Based on that, CMN are usually classified according to their estimated largest diameter in adulthood. Small nevi are up to 1.5 cm, medium are 1.5–19.9 cm and large nevi are those with estimated diameter of more than 20 cm. Giant nevi are 50 cm or larger. Giant nevi are usually accompanied by multiple smaller satellite nevi.
• Congenital nevi present in approximately 1% of births,3 large CMN occur in approximately 1 : 20 000 births,4 and giant lesions (>50 cm) are even less common.5
• While most surgeons are familiar with treating the small and intermediate-size nevi, it is difficult to gain enough experience when approaching more extensive lesions.
• Many strategies have been tried for removal and reconstruction of large and giant nevi. When direct excision and primary closure are not a possibility then tissue expansion is the “workhorse” treatment modality for many medium to large nevi. Facial nevi that cross multiple aesthetic units as well as involving the periorbital area may require expansion in combination with full-thickness skin graft (expanded or nonexpanded). Finally, some unique cases may benefit from a free flap and tissue expansion as an adjunct procedure to close the donor site.
Historical perspective
Initial reports of giant congenital nevus were descriptive: in 1832, a giant nevus was first mentioned in Alibert’s Monograph of Dermatology,6 where it was described as a “waist coat and drawers type naevus.” In 1861 the great Austrian pathologist Karl Rokitansky described a patient with giant congenital nevi in the Allgemeine Wiener medizinische Zeitung (Vienna General Medical Journal).7
Basic science/disease process
The etiology of CMN remains unclear. The development of CMN is determined in utero between the fifth and 24th weeks of gestation. One of the theories of melanocyte differentiation is that, as the neural tube develops during early embryogenesis, melanoblasts migrate from the neural crest along the leptomeninges to the embryonic dermis.6 From the embryonic dermis, the progenitor melanocytic cells migrate into the epidermis, where they differentiate into dendritic melanocytes.
Dysregulated migration, proliferation, and differentiation of melanocytes in the skin and leptomeninges are implicated in the pathogenesis of CMN and neurocutaneous melanosis (NCM).8,9
Several molecular signaling pathways have been associated with the pathogenesis of CMN. Melanocyte development appears partially under the control of c-met and c-kit proto-oncogenes, which encode met and kit proteins, respectively. Hepatocyte growth factor (HGF), also known as scatter factor (SF), is a multifunctional regulator of epithelial cells expressing the tyrosine kinase receptor encoded by c-met. Overexpression of HGF/SF, which is a ligand for the met protein receptor, is implicated in perturbations of melanocyte proliferation, differentiation, survival, and migration.10 Transgenic mice overexpressing HGF/SF are born with cutaneous and leptomeningeal melanocytosis.11 HGF/SF also functions in regulating the migration and differentiation of premyogenic cells during embryogenesis.11 It has been shown that overexpression of this signaling molecule in mice may lead to rhabdomyosarcoma,12 a tumor that on rare occasions also arises in patients with large CMN.13,14 Furthermore, studies with met null mice suggest that met plays a role in NCM, because met knockout mice do not develop NCM.12 Overexpression of HGF/SF and/or met, and sustained activation of met, could explain the mechanism of cutaneous and leptomeningeal melanoma and rhabdomyosarcoma development in individuals with CMN. C-kit, a proto-oncogene that encodes the kit tyrosine kinase receptor for the ligand known as SCF, also plays a role in melanocyte development. In tissue cell culture, c-kit-expressing neural crest cells give rise to clones containing only melanocytes.15 Proliferative nodules, consisting of aggregates of epithelioid or spindled immature benign melanocytes in the dermis of CMN, highly express c-kit.16 Moreover, kit can activate N-RAS, which is an oncogene that is mutated in some cases of nodular melanoma.17 N-RAS mutations have also been reported in CMN,18 suggesting a possible genetic link between CMN and melanoma.
The exact risk for development of melanoma in CMN is not clear. While the relative risk for the incidence of melanoma in patients with large CMN compared to a control group is reported to be high, between 52 and 1046,19,20 the absolute risk is estimated between 1.25% and 10%.20,21 Patients with small and medium-sized CMN have a lower risk of melanoma, with a reported relative risk of 9.545 and an absolute risk between 0 and 4.9%.20,22 Also, many of the articles discussing the issue of relative risk of melanoma do not differentiate between cutaneous and extracutaneous melanoma and the presence of NCM may be the greater risk factor in subsequent risk of malignancy.
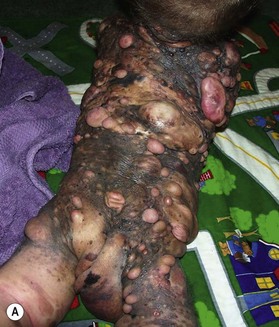
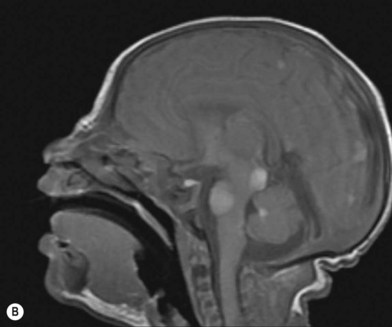
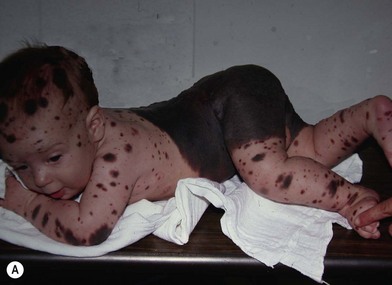
NCM is characterized by an excess deposition of melanocytes along the leptomeninges (Fig. 40.1). It can occur in both patients with large CMN and those with multiple small or medium-sized CMN. Patients with large CMN located on the posterior axis are thought to have greater risk for NCM but, on a multivariate analysis, the only risk factor for NCM in patients with large congenital nevi is having multiple satellite nevi: more than 20 satellites had a 5.1-fold increased risk for NCM compared with patients with fewer satellites.23 The true incidence is not known, but symptomatic NCM may affect 6–11% of patients with large CMN. Symptomatic NCM has a poor prognosis. Symptoms frequently present in early childhood. Neurologic symptoms can manifest themselves as seizures, developmental delay, hydrocephalus, and delayed motor development.
Rarely, other tumors, such as rhabdomyosarcoma and liposarcoma, are associated with CMN.
Diagnosis/patient presentation
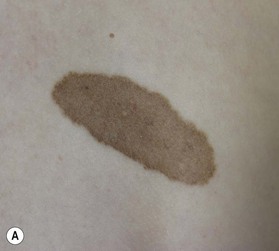
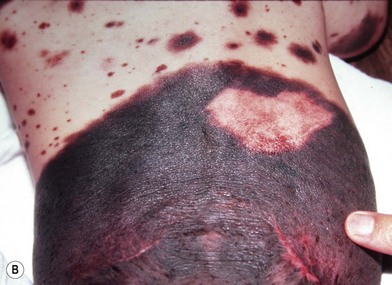
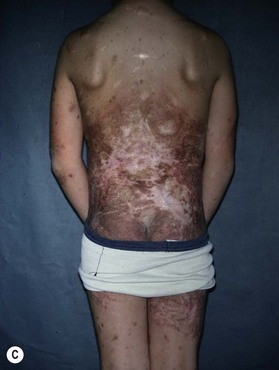
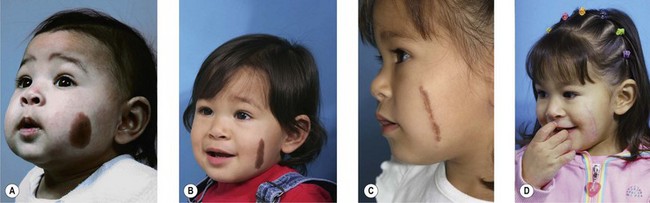
Small to medium-sized CMN usually present as round to oval homogeneous pigmented lesions, light to dark brown in color, with sharply demarcated borders, mamillated surface, and hypertrichosis. However, larger CMN, in particular, may show asymmetry, irregular borders, multicolored pigment pattern, rugous texture, and a nodular surface. In addition, large CMN are often associated with many smaller satellite nevi. As the child grows, especially at puberty, the CMN may change color, becoming lighter or darker, developing hair, becoming more heterogeneous or more homogeneous. CMN may spontaneously regress and some patients may develop vitiligo. Nodular proliferation may be present from birth or develop at a later age. CMN are usually asymptomatic; however, patients with larger lesions may present with pruritus, xerosis, skin fragility, erosions, or ulcerations and decreased ability to sweat from the involved skin (Fig. 40.2).
A review of dermoscopy patterns in congenital nevi found that most nevi demonstrate a reticular, globular, or reticuloglobular pattern. The findings varied with age and the anatomic location of the nevus, with the globular pattern found more often in younger children and the reticular pattern found in patients aged 12 years or older.24
Because of the increased risk of melanoma associated with congenital nevi, attempts have been made to distinguish congenital nevi from acquired nevi on the basis of histology. Distinguishing histologic features include: (1) involvement by nevus cells of deep dermal appendages and neurovascular structures (including hair follicles, sebaceous glands, arrector pili muscles, and within walls of blood vessels); (2) extension of nevus cells to deep dermis and subcutaneous fat; (3) infiltration of nevus cells between collagen bundles; and (4) a nevus cell-poor subepidermal zone.25–27 In contrast to congenital nevi, acquired nevi are usually composed of nevus cells that are limited to the papillary and upper reticular dermis and do not involve the appendages.
In cases associated with a high index of suspicion for the presence of NCM, magnetic resonance imaging of the central nervous system is a useful diagnostic tool (Fig. 40.1B).
Patient selection
The treatment of large and giant nevi is controversial. Although the risk of malignant transformation in congenital pigmented nevi is well established,28–32 many feel that the risk of developing melanoma is too low to warrant the unsightly scars or grafts that may follow treatment. There is no evidence in the literature that demonstrates decrease in occurrence of melanoma after excision of large congenital melanocytic lesion. Furthermore, these patients have an increased risk of extracutaneous melanoma.32,33 Others feel that, in the presence of NCM, the greatest risk lies within the central nervous system, so the excision of the cutaneous lesion can only have limited benefits. However, the appearance of these lesions clearly produces a stigma with significant psychological implications. The challenge for the surgeon involved in treating these often complex lesions is to develop treatment modalities that not only accomplish the excision of all or most of the nevus but also lead to an optimal aesthetic and functional outcome.
Although the lifetime risk of malignant melanoma for small and medium congenital pigmented nevi is reported to be 0–4.9%,34 the risk of melanoma is nearly nil prior to puberty for small nevi,35,36 and so one may comfortably wait until the child is old enough to excise the lesion under local anesthesia. If the lesion is located in an area where the excision and reconstruction may not likely be accomplished under local anesthesia or where there may be the possibility of a better final scar with earlier excision, then early excision under general anesthesia may be warranted. Certainly, many nevi positioned in prominent parts of the face may present as a significant source of peer ridicule starting quite early in the school years and delaying the excision in an effort to avoid a general anesthetic is not in the child’s best interest.
The authors advocate treatment of large and giant nevi by 6 months of age in most cases. Although many of the tissue expansion procedures used in the treatment of large nevi can be applied to older children and adults, the intolerance for repeated procedures and the decreased elasticity of the skin may make the excision of extensive lesions impractical in older patients. Also, for larger nevi the greatest risk for malignancy is in the first few years.37,38
Treatment/surgical technique
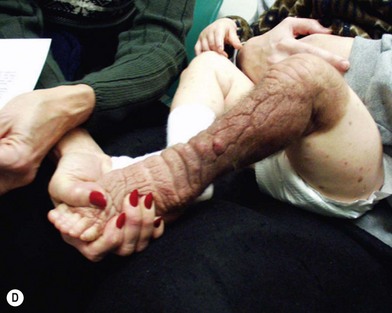
Many strategies have been tried for the removal and reconstruction of large and giant nevi. Serial excision can often debulk these massive lesions but rarely remove them completely. Excision and split-thickness skin graft have generally poor functional and aesthetic outcomes. Dermabrasion, curettage, chemical peel, and laser treatment all have problems with recurrence since these modalities only eliminate the superficial portion of the nevus while the cells of congenital pigmented nevi can usually be found as deep as the subcutaneous fat and sometimes even in deeper structures.29 This group of “partial-thickness” excision, while potentially reducing the overall number of nevus cells and lightening the degree of pigmentation, is commonly associated with later bleedthrough of the deep nevus cells, but may be manifest as both abnormal skin coloration and hypertrichosis (Fig. 40.3). There is also difficulty following the lesions for malignant transformation because of the scarring. The long-term effects of laser treatment on the remaining nevus cells remain to be determined.
Serial excision
Serial excision is the excision of a lesion in more than one stage. The inherent viscoelastic properties of skin are used, allowing the skin to stretch over time. These techniques enable wound closure to be accomplished with a shorter scar than if the original lesion was elliptically excised in a single stage and to reorient the scar closer to the relaxed skin lines. This technique can be applied to small or medium nevi, depending on the location of the nevus and the laxity of the local skin (Fig. 40.4). However, with each stage of the serial excision there is some recoil and serial excision alone, near sensitive areas like the lower eyelid and oral commissure, may create tissue shortage and long-term distortion of structures that would not arise if tissue expansion had been applied rather than serial excision alone.
Excision with skin graft reconstruction
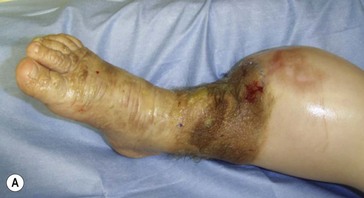
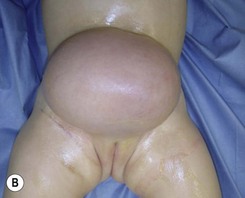
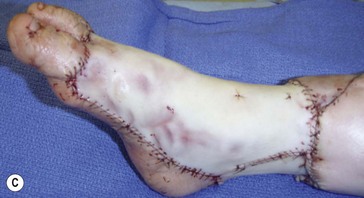
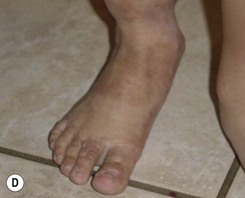
On the face (periorbital region and ear) expanded and nonexpanded full-thickness skin grafts provide good match in both color and thickness for the recipient area. Likewise, expanded full-thickness skin grafts are an excellent choice for coverage on the dorsum of the hand and foot (and distal third of the leg) (Fig. 40.5). However, if the excision is being carried to the fascial level, the contour deformity produced following excision and skin graft to the extremities and trunk can be significant, and result in both aesthetic and functional defects later in life.
Use of split-thickness skin grafting on the trunk, even when done with nonmeshed medium-thickness grafts, can still result in considerable late deformity with potential associated functional defects where the graft skin does not keep up with surrounding growth. The one area of the trunk that can be grafted without significant late contour deformity is the back because of the relatively uniform flat surface. Significant contour deformities develop later where the grafting is carried on to the flanks and anterior trunk (particularly in heavier individuals where the border between grafted and nongrafted skin can create quite dramatic deformities) (Fig. 40.6). Skin grafting of the back can however provide a means of excising a large segment of the nevus, in an area of potentially greater risk of degeneration, and enhance the dermatologist’s ability to map and follow the remaining lesion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree