Collagen XVII has been identified as having a role in inherited junctional epidermolysis bullosa non-Herlitz (JEB-other, MIM #226650). The role of collagen XVII in both autoimmune and genetic blistering disorders demonstrates its relevance to dermal-epidermal adhesion. Collagen XVII is a major structural component of the hemidesmosome (HD), a highly specialized multiprotein complex that mediates the anchorage of basal epithelial cells to the underlying basement membrane in stratified, pseudostratified, and transitional epithelia. This article examines the genetic and pathological features of collagen XVII.
Collagen XVII
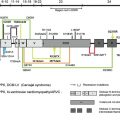
Collagen XVII was initially identified as the 180-kDa bullous pemphigoid antigen (BP180), and several years later its role in inherited junctional epidermolysis bullosa non-Herlitz (JEB-other, MIM #226650) was identified. The role of collagen XVII in both autoimmune and genetic blistering disorders demonstrates its relevance to dermal-epidermal adhesion. Collagen XVII is a major structural component of the hemidesmosome (HD), a highly specialized multiprotein complex that mediates the anchorage of basal epithelial cells to the underlying basement membrane in stratified, pseudostratified, and transitional epithelia ( Fig. 1 A) . Collagen XVII is expressed in skin, oral mucosa, ocular conjunctiva, epithelial basement membrane of the cornea, upper esophagus, transitional epithelium of the bladder, and widely in the brain, located primarily in the soma and proximal axons of neurons. Furthermore, during embryonic development collagen XVII is expressed during the first trimester in syncytial and cytotrophoblastic cells of normal placenta and in epithelial cells of amniotic membranes ; it contributes to embryonic cardiogenesis, regulates ameloblast differentiation, and is essential for normal formation of Tomes’ processes. Sequence analysis for collagen XVII orthologs from other species revealed a particularly high level of evolutionary conservation. For example, at protein level human collagen XVII shows an overall homology of 86% with murine collagen XVII.
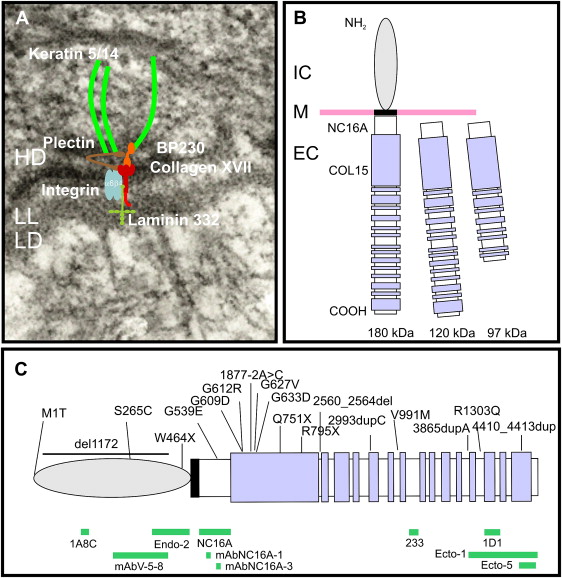
Collagen XVII is a homotrimeric type II transmembrane protein consisting of 3 180-kDa collagen alpha-1(XVII) chains. Each chain is 1497 amino acids long and has a globular N-terminal intracellular domain of 466 amino acids, a short hydrophobic transmembrane stretch of 23 amino acids, and an extracellular C-terminus 1008 amino acids long ( Fig. 1 B). The intracellular domain, part of the HD plaque, has no similarities to other proteins, and interacts with integrin β4, plectin, and BP230. The extracellular domain contains 15 collagen domains. These domains are made up of Gly-X-Y tripeptide repeats with very high proline content at the X and Y positions, and are separated by noncollagenous regions. Collagen XVII extracellular domain contributes to the structure of anchoring filaments in the lamina lucida of the basement membrane, and contains at least one loop structure in the lamina densa. Collagen XVII serves as a cell surface receptor for extracellular matrix proteins; its ligands are laminin 332 and integrin α6 (see Fig. 1 A, B). Further binding partners are still to be identified. The juxtamembranous noncollagenous NC16A region is likely to be important for trimerization and subsequent triple-helix folding in the N → C terminal direction. A particular interest was dedicated to this domain because 80% to 90% of bullous pemphigoid and pemphigoid gestationis patients’ sera targets epitopes within the NC16A domain.
The extracellular domain of collagen XVII can undergo proteolytic processing, resulting in the formation of a 120-kDa fragment (designated as LAD-1), and subsequent cleavage to a second soluble form of 97 kDa (designated as 97-LAD or LABD97) (see Fig. 1 B). The constitutive shedding, which results in the 120-kDa ectodomain, is mediated by ADAM-9, ADAM-10, and ADAM-17 (TACE). This shedding seems to be dependent on the conformation of the NC16A domain and the steric availability of the cleavage site. The regulation is complex: the membrane microenvironment, namely the organization of lipid rafts—cholesterol- and sphingolipids-enriched microdomains within the plasma membrane—may be involved. The extracellular phosphorylation of collagen XVII by ecto-casein kinase 2 is also likely to play a role. The cleavage of collagen XVII occurs in the NC16A domain between the amino acid residues 528 and 547. However, the precise site and the biologic functions of the soluble constitutively shed 120-kDa ectodomain remain elusive. There is evidence that the ectodomain is incorporated in the basement membrane and may have cell adhesion properties. It is also predicted that the cleavage process itself may be important for regulation of keratinocyte detachment from the basement membrane in the process of differentiation and migration. The cleavage of the extracellular domain of collagen XVII, which results in formation of a 97-kDa fragment, seems to be dependent, at least in vitro, on plasmin. The biologic relevance of this is not well understood. Nevertheless, shedding is clinically relevant because the 120-kDa ectodomain (LAD-1) and the 97-kDa form (LABD97) are targets for autoantibodies in the autoimmune disease linear IgA bullous dermatosis.
In vitro experiments demonstrated that absence of collagen XVII has important consequences on cell behavior. Tasanen and colleagues reported that collagen XVII null keratinocytes had a migratory phenotype. The collagen XVII-deficient cells develop lamellipodia on different substrates and show improved spreading on laminin 111, but normal adhesion. This finding implicates collagen XVII in the stabilization of keratinocytes and inhibition of their migration. However, by using another model with different experimental conditions and substrates, Qiao and colleagues showed recently that siRNA COL17A1 knockdown of HaCaT keratinocytes led to reduced adhesion and migration, and implicated the p38MAPK-signaling pathway in this abnormal migratory behavior. Collagen XVII may have a role in the formation of HD through its intracellular domain interacting with HD proteins, and also in the establishment of anchoring filaments through binding its ectodomain to laminin 332, an event that may control cell motility.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree